
Experimental Cancer Drug Metarrestin Targets Metastatic Tumors
May 29, 2018, by NCI Staff
Most deaths from cancer are caused not by the original tumor, but by the physiologic toll of tumors that have spread, or metastasized, to other parts of the body.
But a new study, conducted largely in mouse models of cancer, suggests that an experimental drug may offer a way to address this critical problem. In the study, the drug showed that it can do what other therapies have been unable to accomplish: selectively attack metastatic tumors.
In mice with pancreatic cancer that had spread to other organs, treatment with the drug—called metarrestin—shrank metastatic tumors and greatly extended how long the mice lived. The drug appeared to be generally safe, with no evidence that it harmed the organs where metastatic tumors had formed or the mice in any way that the researchers could measure.
Metarrestin also shrank metastatic tumors in models of breast and prostate cancer, the researchers reported May 16 in Science Translational Medicine.
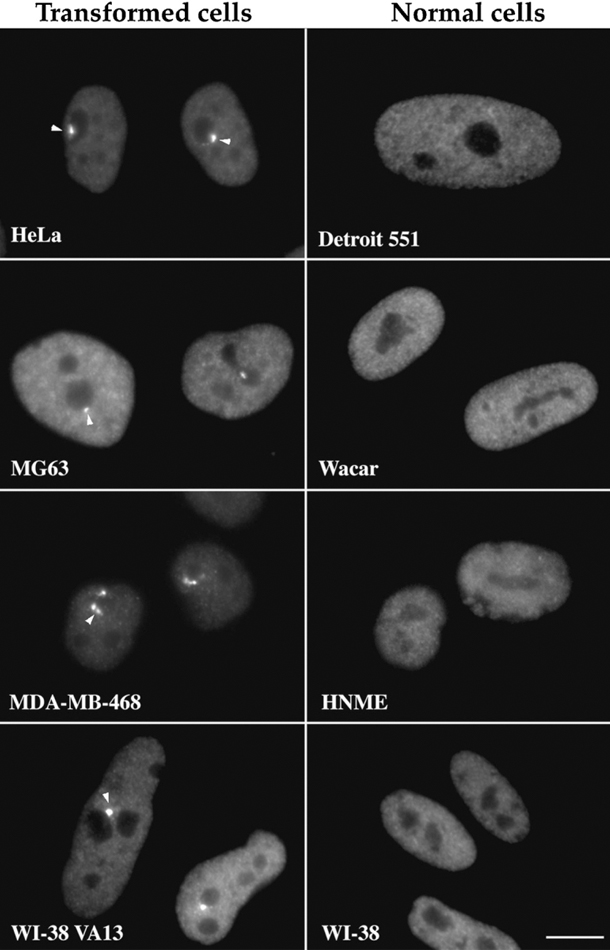
The drug appears to work by killing cells that have developed a structure called the perinucleolar compartment (PNC) within their nuclei. These structures develop almost exclusively in cancer cells—especially the cancer cells in metastatic tumors—and not in healthy cells, explained one of the study’s lead investigators, Sui Huang, M.D., Ph.D., of Northwestern University's Feinberg School of Medicine. Dr. Huang was the first to associate PNCs with cancer, nearly 25 years ago.
Although there are some clues, there are still unanswered questions about what PNCs do in cancer cells and exactly how metarrestin works, stressed Udo Rudloff, M.D., of NCI's Center for Cancer Research and one of the study’s lead investigators.
But the effectiveness and safety that metarrestin displayed in the study “is very exciting,” Dr. Rudloff continued, and efforts to move the drug into clinical trials are already underway.
Targeting a Marker of Metastasis
How cancer metastases form and grow continues to be poorly understood, Dr. Huang said, and there has been little progress in developing treatments that specifically target metastatic tumors.
A major obstacle to progress is the tremendous complexity of metastatic tumors, she continued, which are filled with cancer cells that are riddled with genetic and nongenetic alterations. However, she added, metastatic cancer cells do have specific characteristics that differentiate them from normal cells and the other cancer cells in the primary tumor.
Among these characteristics are PNCs. In addition to being ubiquitous in metastatic cancer cells, PNCs also have been linked with poor survival in several different cancer types.
Taken together, the available data on PNCs suggests that their formation "reflects the complex changes of metastatic cancer," Dr. Huang explained.
Many studies try to identify a single genetic alteration commonly found in cancer cells and then develop a therapy that targets that alteration. The "fundamental idea" of this new study, she said, was to use PNCs as a marker of the metastatic behavior of cancer cells and test whether compounds that disrupt the marker could potentially derail the metastatic process.
A Long, Collaborative Effort
The identification of metarrestin began nearly a decade ago and involved a collaboration between Dr. Huang’s lab at Northwestern and researchers at the University of Kansas and NIH.
The initial work was performed by investigators at NIH’s National Center for Advancing Translation Sciences (NCATS), who developed a "screen" for systematically testing compounds—approximately 140,000 in total—in a prostate cancer cell line rife with PNC-containing cells. From that intensive process, they identified a compound that was highly effective at reducing PNC prevalence in the cells and that had other favorable features of a potential metastasis-blocking therapy.
Next, researchers from the University of Kansas and the NCATS Chemical Genomics Center, led by Juan Marugan, Ph.D., and a co-lead investigator on the study, formulated derivatives of the compound—approximately 150 different formulations to arrive at one with the greatest potency and other properties needed for a drug to work well in the human body.
The final optimized compound was named metarrestin.
After experiments in cancer cell lines confirmed that the experimental drug could effectively reduce PNC prevalence and kill cancer cells, the researchers moved to studies in mouse models.
In work led by Dr. Rudloff and his NCI colleagues, the drug was first tested in a mouse model of pancreatic cancer that closely mimics how the cancer behaves in humans and that the researchers confirmed had high PNC prevalence in metastatic tumors.
In the mice, metarrestin treatment dramatically reduced PNC prevalence in metastatic tumor cells and more than doubled how long the mice lived. Whereas untreated mice had tumor-ridden livers and lungs, these organs were completely free of metastases and appeared to be perfectly normal in mice that had received metarrestin. In addition, there was no evidence of any side effects from the treatment.
And even though PNCs can also be detected in cancer cells in primary tumors, metarrestin seemed to mostly affect metastatic tumors.
"There was no significant difference in primary tumor growth in the pancreas between treated and untreated animals," the researchers wrote. "These findings suggest a survival gain due to suppression of metastasis-related death."
PNC Function and Metarrestin Mechanism
PNCs usually form at the periphery of another structure within the nucleus, called the nucleolus, where ribosomes are built. Ribosomes are protein factories—building, or synthesizing, the proteins that cells need to carry out essential functions and pumping them out into the cell.
Dr. Rudloff said it's likely that the PNCs play a role in regulating molecular machinery that make ribosomes. And in metastatic cancer cells, ribosomes have a lot of work to do, producing the abundance of proteins the cells need to survive and grow.
Findings from additional experiments using cancer cell lines suggest that metarrestin may work by disrupting this process. Those experiments, the researchers reported, showed that metarrestin treatment caused the nucleolus to "collapse."
Additional experiments led them to a protein called eEF1A2, which is involved in the formation of ribosomes. When the researchers experimentally blocked eEF1A2 in cancer cells, it largely mimicked what they saw in metarrestin-treated cancer cells.
Even with these findings, exactly what the PNC does in cancer cells is still to be determined, Dr. Huang cautioned. "But it's clearly a manifestation of the complex multi-step transformation from a normal cell to a cancerous one," she said.
Plans for Human Trials Underway
"Our results show metarrestin is a very promising agent that we should continue to investigate against metastasis," said Dr. Marugan.
In fact, with help from NCATS's Bridging Interventional Development Gaps program, Dr. Rudloff is working with NCATS colleagues to move metarrestin into human trials.
The researchers have created a pill-based form of metarrestin and are collecting the laboratory data on it they need to submit an Investigational New Drug application to the Food and Drug Administration to begin testing the drug in clinical studies.
If all goes as planned, Dr. Rudloff said, the application should be filed by this fall or early 2019. Human trials would begin at NIH Clinical Center soon after, he explained, initially including patients with any type of metastatic solid cancer, and thereafter with a special focus on pancreatic cancer.
No hay comentarios:
Publicar un comentario