
New approach for easier detection of SARS-CoV-2 via swabs and saliva
A recent Israeli study points toward Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) as a potentially effective tool for acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on-the-spot detection by using swabs or saliva – addressing the community need for simple surveillance. The research titled 'SARS-CoV-2 On-the-Spot Virus Detection Directly From Patients' is published on the preprint server medRxiv.
Since emerging in China in December 2019, the coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has progressed to a pandemic very swiftly. The virus is transmitted via direct or indirect contact, droplet spray in short-range, and airborne (aerosol) in the long-range. Due to such ease of spread, many countries worldwide are currently in a lockdown state.
Colorized scanning electron micrograph of an apoptotic cell (blue) infected with SARS-COV-2 virus particles (yellow), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
Human to human transmission mainly occurs via saliva droplets; consequently, a higher viral load is found in the saliva when compared with standard swabs. This is why the U.S. Food and Drug Administration (FDA) has recently approved saliva as a possible sample for COVID-19 testing.
"We believe that a simple and easy detection method, preferably one that can be performed and interpreted on-the-spot, could relieve some of the current limitations and help execute an efficient and safe exit strategy from lockdowns", say the authors of this new study from Israel and Canada.
The limitations of current diagnostic approaches
Detection of viral ribonucleic acid (RNA) in patients is a current gold standard to detect SARS-CoV-2, which is mostly performed at hospitals and public health institutes by professionals. Unlike antibody detection procedures, identifying viral RNA represents a direct measure to precisely appraise whether the person is contagious.
But amidst the ongoing COVID-19 pandemic, it is evident that the availability and performance yield of standard methods is limited both by accessibility and required resources. Furthermore, these methods necessitate RNA purification, followed by reverse transcription and quantitative polymerase chain reaction (RT-qPCR), which is both resource and time-consuming.
However, some alternative molecular methods can bridge these limitations. One of them is colorimetric Loop-Mediated Isothermal Amplification (LAMP) that allows a one-step reverse transcription, enables visualization by color change, and eliminates the need for sophisticated laboratory equipment.
This is why the researchers from the Rambam Health Care Campus (RHCC) were interested in interrogating the potential of RT-LAMP as a direct method to detect viral presence in suspected COVID-19 patients. Previous research has shown that RT-LAMP assays do not show cross-reactivity to other coronaviruses.
Rapid and straightforward way to detect RNA
This research has demonstrated that RT-LAMP is a rapid and straightforward method to detect purified RNA of SARS-CoV-2. By studying samples from COVID-19 positive and negative patients, the scientists adjusted RT-LAMP for the detection of SARS-CoV-2 nucleic acid directly from human patient swabs – without the need for additional RNA purification steps.
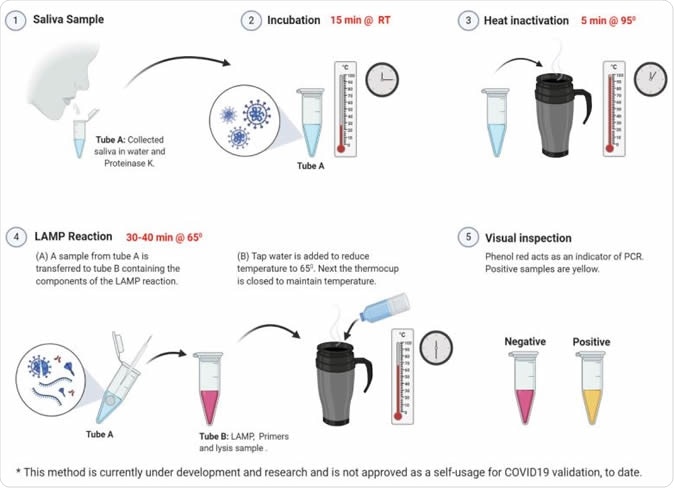
Applying the RT-LAMP protocol on saliva samples. RT-LAMP tests on saliva from 4 volunteers. Each tube represents one tested volunteer. Results of t=0 and t=35 are shown. Upper panel, RT-LAMP reaction using POP7 primers as a positive control. Middle panel, RT-LAMP reaction with no primers control. Lower panel, RT-LAMP reaction using SARSCoV-2 gene N primers. The same samples were analyzed by the conventional hospital RT-qPCR protocol. The RT-qPCR results and Ct values are shown under the relevant samples. b, Graphical illustration of the potential of RT-LAMP protocol to perform self-saliva testing.
For comparison purposes, the experiments were performed simultaneously with the standard RT-qPCR method at the RHCC hospital. Afterward, RT-qPCR cycle threshold values were compared to the RT-LAMP results of more than 180 different patients.
After calibration, it was shown that this type of direct RT-LAMP method usage could successfully detect patients with medium or high viral loads, with very few false-positive results. Moreover, the lack of RNA purification step is a significant move towards scaling-up, as only a constant heat source is needed (such as the thermal mug).
Self-collected saliva samples are also shown to be a valid specimen for this technique. The patients harboring SARS-CoV-2 were also positive in both RT-qPCR and RT-LAMP from saliva, while the suspected negative subject was confirmed negative by using saliva specimen.
Finally, the researchers have tested samples from patients infected with viruses other than SARS-CoV-2 (such as influenza virus, respiratory syncytial virus, herpes simplex virus, and enterovirus). It was shown that all RT-LAMP reactions in those patients were negative.
A real-world application
"Upon such further development, such SARS-CoV-2 detection method can be applied as a surveillance tool for sampling larger populations of the community", study authors highlight the importance of their findings. And indeed, its potential availability and low cost enable continuous monitoring of suspected individuals.
"With further development, this method can be applied to medical clinics, points of entry, nursing homes, workplaces, etc.", state the authors of the study. "Importantly, this method can be easily adjusted to other emerging pathogens as well", they conclude.
Their protocol takes about one hour from sampling to final detection, with the use of very few reagents; also, the procedure can potentially be self-performed or carried out by non-professionals. Such hallmarks may allow its swift implementation worldwide (including in rural regions) after additional studies confirm its potential.
Journal reference:
Ben-Assa, N. (2020). SARS-CoV-2 On-the-Spot Virus Detection Directly from Patients. medRxiv https://doi.org/10.1101/2020.04.22.20072389

































No hay comentarios:
Publicar un comentario