
Innate Resistance to HIV through Natural Killer Cells
Natural killer (NK) cells are large granular lymphocytes that possess natural cytotoxicity against tumor cells and virus-infected cells, as well producing cytokine signal messengers.
NK cells are part of innate immunity, and thus are intrinsically able to differentiate target cells to be destroyed from healthy cells that carry self-antigens. In addition to this, researchers have discovered other capabilities of NK cells that are similar to the adaptive immune response, such as memory of previous encounters with infections and responding with an adaptive immune reaction. This feature shows promise of exploitation through vaccine in diseases like HIV.
Anatomically correct HIV Virus floating in the bloodstream. 3D Illustration. Image Credit: Spectral-Design / Shutterstock
NK Cell Actions
The activating NK cell receptors lead to the recognition of cell surface ligands that appear on cells which are under attack in some way e.g. an invading virus. This may include the self-ligands induced by stress, non-self ligands induced by infection, and Toll-like receptor (TLR) ligands. For instance, when NK cells come into contact with TLR ligands the result is increased cytotoxicity and decreased production of interferon-gamma.
Another receptor is the Fc receptor CD16 which promotes the recognition of target cells which are coated with antibody to cause antibody-dependent cell cytotoxicity (ADCC).
Inhibitory receptors, on the other hand, detect the absence of self-molecules which are constitutively expressed on target cells. Specific receptors on the NK cells recognize MHC I antigens on other cells.
The absence of these is interpreted as ‘missing self’, as may happen with cells under stress, and such cells are targeted for cytotoxicity by the NK cells. In this way NK cells show tolerance to self-molecules while being toxic to stressed cells. NK cells are found throughout the lymphoid and non-lymphoid tissues.
NK cells are of different types, based on whether they express CD56 and CD16. Both are involved in immunity against viral infection. NK cells with strong CD56 expression but absence of CD16 receptors form only a small subset in healthy individuals, with restricted cytotoxicity but high cytokine production.
Most NK cells in circulation have low CD56 expression, proliferate slowly, have strong cytotoxic capability and the ability to acquire killer immunoglobulin-like receptors (KIRs), as well as other cell markers progressively as they differentiate. They also have a range of other receptors such as natural cytotoxicity receptors, signaling lymphocyte activation (SLAM) receptors and C-type lectin receptors.
NK Cells and HIV
Infection of HIV RNA into a cell occurs via viral envelop glycoprotein Env attaching to own-cell surface glycoproteins CD4 and either CXCR4 (C-X-C chemokine receptor 4) or CCR5 (C-C chemokine receptor 5). Once inserted into the host cell, HIV-1 viruses need to escape immune surveillance at a long series of checkpoints to be able to replicate and survive within an immunocompetent host.
This is done via a number of mechanisms, one of which, shown in vitro, involves the virus acting to reduce the cytotoxic activity of NK cells towards infected cells by downregulating their cell surface MHC class I expression and therefore rendering NK cells unable to recognize their invasion.
A second results in the decreasing the priming effect of NK cells on other effector immune cells in the adaptive immune system and a third way it escapes elimination is by infecting a subpopulation of NK cells that are susceptible to the virus. These mechanisms mean that the body finds itself unable to fight the virus efficiently.
NK cells can suppress the replication of HIV viruses within CD4+ lymphocytes efficiently, primarily by the secretion of CC-chemokines. This suppression is independent of interferon-gamma secretion and doesn’t require cytolytic activity. The greater the viremia, however, the lower the level of CC-chemokine secretion.
During the primary phase of a HIV infection, the start of treatment with antiretroviral therapy (ART) is marked by an increase in NK cells expressing CD56 with a decrease in CD16+CD56dim cells at the same time.
Other molecules which mark alterations in immunity also change with the beginning of treatment such as galectin 9 and TIM-3, associated with increased NK cell activity.
However, their interaction in the chronic phase of HIV may lead to dysfunction of NK cells. When TIM-3 is not properly expressed on NK cells the CD4 cells fail to recover well following treatment, indicating that NK cells are essential to immune recovery in at least a subset of patients.
Chronic infection also leads to an increase in the number of CD56-CD16+ cells which have low ADCC, while a fall in viral numbers in the blood leads to restoration of normal ADCC activity.
On the other hand, ART causes NK cells to show less antibody-conferred activity, which must be also handled properly to achieve the full effectiveness of this cellular immune defense.
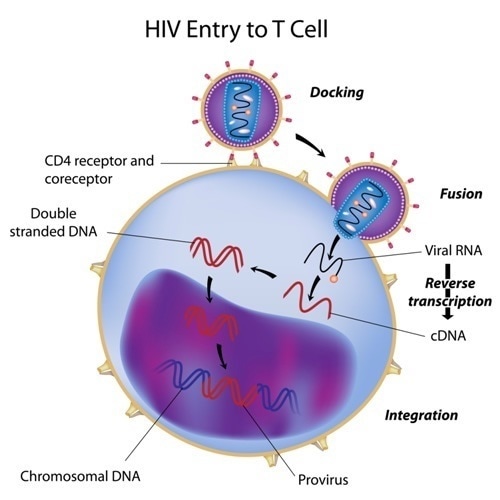
HIV entry to T cell. Image Credit: Alila Medical Media / Shutterstock
Genetic Evidence
Research has been conducted exploring protective effects of certain genotypes to HIV infection and studies support a protective function of NK cells in the beginning phases of HIV infection. KIRs are found on NK cell surfaces to aid the control of activation and inhibition of NK-cell reactions and alleles of this receptor have been closely linked to the speed of progression of HIV to AIDS.
A study carried out by M.P Martin and colleagues found that individuals who had HLA-Bw4-80Ile alleles, as well as the KIR3DS1 allele had a slower progression of HIV to AIDS. The group also found that not having the KIR3DS1 allele increased the development of HIV to AIDS.
Future Treatments
Knowing how NK cells will react to treatment is essential to planning effective therapy in a variety of patients. This requires the study of the receptor expression on the surface of NK cells, such as NKG2A receptors which produce effects such as degranulation, interferon-gamma release and CCL4 production at high levels when exposed to infected CD4+ cells. Some HIV subtypes, on the other hand, cause NK cell dysfunction.
Some work indicates that certain vaccines are capable of restoring NK-mediated interferon-gamma release which results in the lysis of infected CD4+ cells, by NK activation as well as other pathways mediated by signals from recruited NK cells.
Proper direction of the interaction between adaptive immune cells and NK cell responses can help make use of effective NK cell activation pathways. The restoration of this powerful immune response may help to achieve eradication of the virus and cure of the infection with customized ART in the future, especially when the immune system shows signs of dysfunction and anergy.
Sources
Further Reading
Last Updated: Oct 22, 2018

































No hay comentarios:
Publicar un comentario