
High-Fat Diet or Diabetes Drug May Enhance Response to Targeted Cancer Drug
August 8, 2018, by NCI Staff
A new study in mice suggests two different ways to overcome treatment resistance to—and improve the efficacy of—targeted cancer drugs known as PI3K inhibitors. Resistance to these drugs, the researchers showed, appears to be caused by increases in insulin, a hormone that regulates glucose and plays an important role in the development of diabetes.
In the study, mice given a PI3K inhibitor along with a high-fat (ketogenic) diet or a commonly used diabetes drug had greater tumor shrinkage than mice given the cancer drug alone.
The results of the NCI-funded study were reported in Nature on July 4.
The study “is important because it shows the complexity of targeting PI3K for cancer therapies and explains why PI3K inhibitors can cause different responses in clinical trials,” said Rihab Yassin, Ph.D., of the Cancer Cell Biology Branch in NCI’s Division of Cancer Biology.
“The findings also suggest that resistance to PI3K inhibitors in many tumors could be counteracted with dietary or drug interventions,” Dr. Yassin added.
Targeting a Driver of Cancer Growth and Metabolism
The study’s lead author, Lewis C. Cantley, Ph.D., of Weill Cornell Medicine, discovered the PI3K enzyme about 30 years ago and showed that it is a driver of many cancers. In fact, genetic mutations that increase PI3K activity are among the most frequent genetic changes found in cancers.
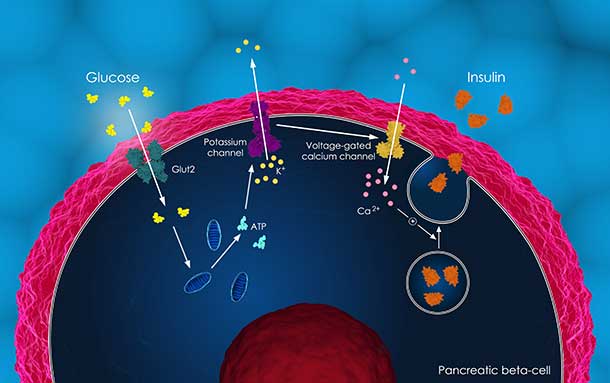
Around 1990, Dr. Cantley found that PI3K stimulates cells to take up glucose in response to insulin. Cancer cells often increase the activation of PI3K because they rely on abnormal glucose metabolism for rapid growth, meaning they use more glucose to quickly generate the cellular building blocks of protein and DNA.
The Food and Drug Administration has already approved two PI3K inhibitors for the treatment of leukemia and lymphoma. But in clinical trials of people with solid cancersPI3K inhibitors have not been shown to improve how long people live, spurring researchers to investigate why this happens.
Drug Resistance Through a Feedback Loop
Understanding and overcoming drug resistance continues to be a major challenge for cancer researchers. In one form of resistance, a cancer drug causes biological effects that counteract how the drug works to kill cancer cells—that is, its mechanism of action.
Early clinical trials of PI3K inhibitors demonstrated that these drugs can increase patients’ blood glucose levels. Dr. Cantley and his colleagues wanted to see if this spike in glucose generated a feedback loop that blocked the action of PI3K inhibitors and caused treatment resistance.
That’s exactly what they found. In mouse models of cancer, they confirmed that PI3K inhibitors increase glucose blood levels by blocking the cell’s ability to take up glucose. As glucose levels rise, the body releases insulin from the pancreas to keep glucose levels in check. The increase in insulin, the researchers showed, caused cancer cells to override the PI3K inhibitor and activate a PI3K signaling loop that supports cancer cell survival and growth.
Improving Responses to PI3K Inhibitors with Diet, Diabetes Medication
The researchers then decided to test whether blocking insulin feedback by decreasing blood levels of glucose and insulin might improve the effectiveness of PI3K inhibitors.
They studied PI3K inhibition in combination with a ketogenic diet, which consists of mainly fats, a moderate amount of protein, and very few carbohydrates.
“This [diet] forces the body to burn fat and keeps body weight, glucose levels, and insulin levels low,” Dr. Cantley explained.
Experiments in different mouse tumor models—including pancreatic, bladder, endometrial, and breast cancers—showed that feeding the mice a ketogenic diet improved their responses to PI3K inhibitors and reduced tumor growth. The improved efficacy was likely due to a decrease in insulin and its ability to re-activate PI3K in the tumor cells, the researchers wrote. Dr. Cantley also emphasized that, unlike what’s been seen with some other combinations of cancer therapies, use of the ketogenic diet with PI3K inhibitors didn’t appear to cause any additional side effects in the mice.
A ketogenic diet alone “had variable effects” in the different tumor models used in the study, the researchers wrote, “indicating that the dietary changes themselves were insufficient to cause the tumor responses observed.” In fact, in a mouse model of leukemia, the diet appeared to accelerate cancer progression.
The researchers also showed that giving mice insulin after PI3K-inhibitor treatment counteracted the ketogenic diet’s effects and caused tumors to begin growing.
Dr. Cantley noted that more research is needed to understand the effects of diet on cancer progression and responses to treatment. However, he thinks that these findings suggest that the ketogenic diet may help certain cancer patients being treated with PI3K inhibitors.
The findings in mouse models of pancreatic cancer were particularly intriguing, Dr. Cantley said.
“We [have] never observed responses to PI3K inhibitors in mouse models of pancreatic cancer, since this type of cancer rarely has PI3K mutations,” he said. Yet, when the mice were put on a ketogenic diet in combination with PI3K inhibitors, their tumors shrank significantly.
In addition to dietary changes, Dr. Cantley’s group also examined whether two blood glucose-lowering drugs commonly used to treat diabetes, metformin and SGLT2 inhibitors, could disrupt the PI3K-related insulin feedback loop.
Giving mice metformin before PI3K inhibitor treatment didn’t significantly lower blood glucose and insulin levels or block PI3K signaling. SGLT2 inhibitors, on the other hand, suppressed insulin feedback and the reactivation of PI3K.
SGLT2 inhibitors work in a different way from other diabetes drugs and are particularly effective in reducing glucose and insulin levels, explained Dr. Cantley. That may be why they were more successful than metformin at blocking tumor growth in combination with PI3K inhibitors in this study, he added.
Yet, the most effective approach to reduce the insulin levels of the mice in the study was the ketogenic diet. As a result, Dr. Cantley said he is exploring a clinical trial with drug companies to investigate combining a ketogenic diet with a PI3K inhibitor in patients with lymphoma or endometrial cancer.





















.png)










No hay comentarios:
Publicar un comentario