
Scientists reverse congenital blindness in mouse model
A study funded by the National Eye Institute (NEI) has shown that congenital blindness in mice could be reversed by regenerating rod photoreceptors in the retina.
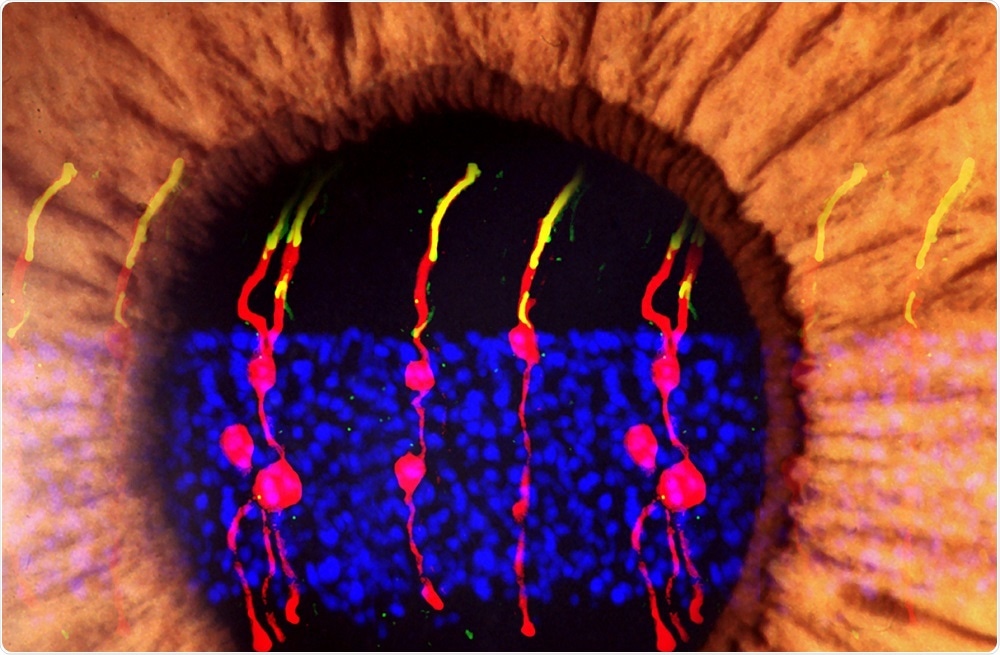 Image Credit: Bo Chen, Ph.D
Image Credit: Bo Chen, Ph.D
The findings have important implications for research into regenerative therapies for diseases such as age-related macular degeneration and retinitis pigmentosa.
As reported in the journal Nature, researchers turned supportive cells called Müller glia into functional rod photoreceptors that could integrate with other types of neurons in the retina and brain.
The Müller glia have long been studied for their regenerative potential because they have previously been shown to undergo division in response to injury and change into photoreceptors and other neurons in the retina.
However, this has only been demonstrated in non-mammalian species such as zebra fish and scientists have found that to prompt mammalian Müller glia to behave similarly to how they do in fish, they need to injure the tissue first.
To achieve this, Professor Bo Chen and colleagues first induced division of Müller glia in healthy mice by injecting a gene into the eye that activates a protein called beta-catenin.
Once the cells had divided, factors that prompt the cells to turn into rod photoreceptors were injected into the eyes and the new rods were tracked using microscopy.
Chen and team report that the regenerated rod photoreceptors appeared to have the same structure as naturally occurring photoreceptors.
They had also formed synaptic structures that enabled them to communicate with other retinal neurons.
To test whether the newly formed rods were functional, the researchers applied the approach to blind mice that had been born without functional rods.
They found that the rods derived from Müller glia developed just as well as they had in the healthy mice and were communicating with other types of neurons in the retina across synapses.
Further tests showed that the Müller glia-derived rods were integrated into the visual pathway circuitry connecting the retina and the brain’s primary visual cortex.
Next, the team plans to conduct studies that will show whether the mice have regained the ability to complete visual tasks and to test the effectiveness of the approach in human retinal tissue.






















.png)










No hay comentarios:
Publicar un comentario