
Function and Characteristics of Astrocytes in Disease and Development
Characteristics of Human Astrocytes
Astrocytes, also known as astroglia, are characterized by their star-like shape and are the most abundant cell type in the brain. They have important roles in the blood-brain barrier function and in synaptic activity, and are closely linked to neurons. The study of astrocytes using in vitro co-culture models is becoming more important in neuroscience research.
Astrocytes support the trophic and metabolic development of neurons. As they mature, these cells have a broad range of functions in myelination, neuronal migration and synaptogenesis. These functions are well-established and stage-specific.
Compared to individual rodent astrocytes, human astrocytes have a vastly increased number of synapses and are more than 20 times larger by volume.
Astrogenesis
The three major cell types in the developing neuronal system, astrocytes, oligodendrocytes and neurons, share common ancestors. These are progenitor cells or neural stem cells. During the initial phase of neuronal development, neural stem cells contribute to neurogenesis which is followed by a wave of gliogenesis. The JAK/STAT and Notch are two of the major signaling pathways which are known to contribute to activation of astrogenesis. These pathways are activated by upstream extrinsic factors. The main components are CNTF, CT-1, members of the IL-6 cytokine family and LIF. Along with BMP and Notch signaling, these lead to astrogenesis stimulation.
One major consequence of these factors is the activation of STAT3. It is critical to the induction of expression of astrocyte-lineage specific genes at the transcriptional level. Two such genes are S100B and GFAP. Furthermore, STAT3 is an essential component of the transcriptional complex STAT3:p300/CBP:SMAD1, which modulates activation factors involved in astrogenesis. The process also involves other transcription factors, for example HES1 and SOX9. Furthermore, in order for astrocyte development to proceed, chromatin modifications, for example demethylation of the GFAP promotor, must occur.
Astrocytes in Human Neurodevelopmental Disorders
It is becoming more obvious that astrocytes are very important when the choreography of neural development is disrupted. This disruption leads to pathogenesis of neurodevelopmental disorders.
Understanding gained from monogenetic forms of autism has demonstrated that these disorders can be underlined by primary astrocyte dysfunction. Rett syndrome, for example, is caused by mutations in methyl CpG binding protein 2 (MECP2) and features altered synapse physiology and abnormal dendritic morphology. Increased levels of glial fibrillary acidic protein (GFAP) are expressed by patients with schizophrenia, and this is a feature associated with astrogliosis.
It is very important to understand the impact of astrocyte dysfunction and behavior, including the impact on surrounding neurons. This is critical not only for understanding the underlying causes of these disorders, but also for creating future therapies.
Astrocytes and Neurodegeneration
For a long time, Parkinson’s and Alzheimer’s diseases, and other neurodegenerative disorders, have been investigated from the perspective of neuronal degeneration. Evidence suggests that often, glial atrophy appears at early stages of the disease. In amyotrophic lateral sclerosis (ALS), for example, degeneration of astrocytes occurs before symptoms caused by motor-neuron defects. Furthermore, astrocyte activation has been linked to amyloid beta production in Alzheimer’s disease and so contributes towards the formation of amyloid plaques.
Other Potential Areas of Study
- Neurogenesis research
- Study of astrocyte-mediated neurotoxicity
- Glial scar formation
- Study of inflammation and healing in the brain
- Blood-brain barrier
- Neuron and astrocyte co-culture to examine surface molecule expression and trophic factor release
- Stroke
- Drug development for neurodegenerative disorders
Human Induced Pluripotent Stem Cell (iPSC)-Derived Astrocytes in Disease Modeling and Drug Discovery
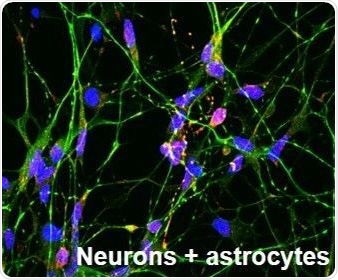
Researchers can generate pure cell populations for use in disease modeling and drug discovery using human induced pluripotent stem cells (iPSC)-derived astrocytes and culture reagents, such as those offered by Axol.
Cultures such as these are biologically relevant and could be used to elucidate the mechanisms that occur in neuronal development or precede the onset of neurological conditions, or to determine the direct effect of compounds on these specific cells.
They can also be co-cultured in order to perform more complex analysis of the central nervous system (CNS) in vitro. By using technologies such as CRISPR/Cas9 to generate isogenic cell lines with disease-specific mutations, further precision may be achieved. This means that cells can be directly compared, without the influence of confounding genetic or environmental factors that may be present in samples derived from individuals.
Astrocyte Immunocytochemistry (ICC) Markers
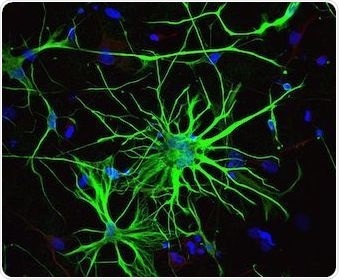
It is critical that the in vitro differentiation of iPSCs mimics the in vivo process. In order to determine the developmental stage of the cell therefore, marker expression should be examined. The prototype marker used to confirm astrocyte phenotype and to differentiation them from other glial cells, is GFAP.
Astrocytes will have high levels of GFAP and low levels of a microtubule marker of neuronal lineage known as neuronal class III ß-tubulin (TUJ1). Typically, iPSC-Derived Astrocyte populations from Axol contain £ 15% TUJ1-positive neurons and ³ 80% GFAP-positive astrocytes.
References
- Role of glial cells in the formation and maintenance of synapses. Pfrieger et al., 2010
- Astrocytes and disease: a neurodevelopmental perspective. Molofsky et al., 2012
- Uniquely hominid features of adult human astrocytes. Oberheim et al., 2009
- Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Sloan & Barres, 2014
- Potentiation of astrogliogenesis by STAT3-mediated activation of bone morphogenetic protein-SMAD signaling in neural stem cells. Fukuda et al., 2007
- A positive autoregulatory loop of JAK-STAT signaling controls the onset of astrogliogenesis. He et al., 2005
- Synergistic effect of retinoic acid and cytokines on the regulation of glial fibrillary acidic protein expression. Herrera & Schubert, 2010
- Interplay between SIN3A and STAT3 mediates chromatin conformational changes and GFAP expression during cellular differentiation. Yu et al., 2011
- Developmental maturation of astrocytes and pathogenesis of neurodevelopmental disorders. Yang et al. 2013
- Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. Catts et al., 2014
- Neuroglia in ageing and disease. Verkhratsky et al., 2014
- The contribution of activated astrocytes to AB production: Implications for Alzheimer's disease pathogenesis. Zhao et al., 2011
- Astrocytes in Alzheimer's disease. Verkhratsky et al., 2010
- GFAP glial fibrillary acidic protein [Homo sapiens (human)]. NCBI http://www.ncbi.nlm.nih.gov/gene/2670
































No hay comentarios:
Publicar un comentario