
Study Tests Immunotherapy in People with Cancer and Autoimmune Diseases
, by NCI Staff
Researchers have launched a clinical trial to test an immunotherapy drug in patients who have both cancer and an autoimmune disease, such as rheumatoid arthritis, lupus, or multiple sclerosis.
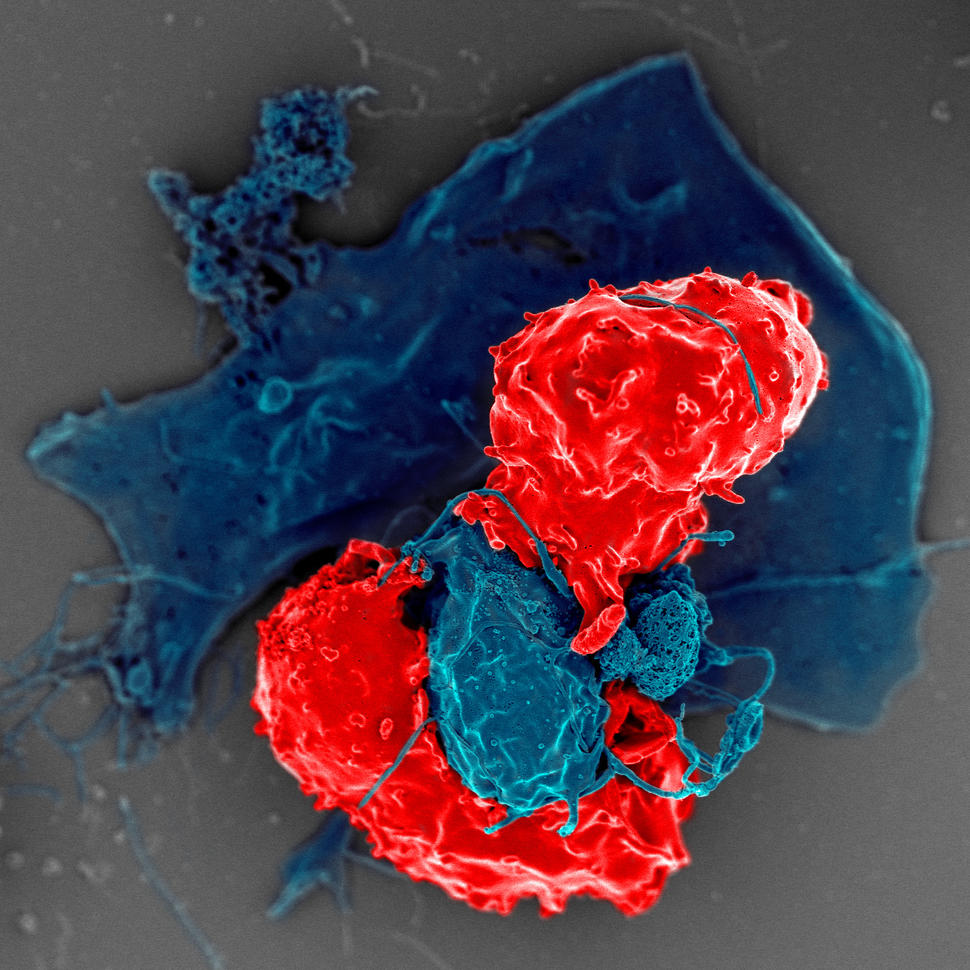
Immunotherapy drugs enhance the ability of the immune system to detect and kill tumor cells. In recent years, these therapies have benefited a growing number of patients, including some patients with advanced cancers.
But doctors have not known whether immunotherapy is safe and effective for people who have both cancer and an autoimmune disease, because such patients have been excluded from clinical trials of immunotherapy drugs.
“Having an overactive immune system is the main reason that many patients with both cancer and autoimmune diseases have not been included in hundreds of clinical trials of immunotherapy drugs,” said Hussein Tawbi, M.D., Ph.D., of the University of Texas MD Anderson Cancer Center and one of the lead investigators for the new trial.
For many doctors who treat patients with both diseases, the lack of information on the effects of immunotherapy in such patients has led to a “clinical conundrum,” Dr. Tawbi continued.
“As doctors, we have been afraid of using these drugs in patients with autoimmune diseases because we don’t have evidence that they are safe for these patients,” he added. “There’s no guidance on how to handle these cases.”
The main concerns, Dr. Tawbi explained, are that “the immune-related side effects may be more severe in patients with both diseases, or that their autoimmune conditions may get much worse because of immunotherapy.”
In addition, because these patients have been excluded from clinical trials, doctors don’t know “whether they may respond better [than other patients]—or possibly worse because they could be on therapy to suppress the immune system,” he added.
The new clinical trial, which is sponsored by NCI, is intended to help researchers understand the potential harms and benefits of using immunotherapy in patients with certain autoimmune diseases.
The trial could also yield insights into the biology of autoimmune diseases, which could help researchers explore new treatments, according to Dr. Tawbi.
“This study is the first of its kind,” he said. “What we learn may allow us to extend the promise of immunotherapy in a safe manner to patients who have cancer and an existing autoimmune disease.”
Between 10% and 30% of cancer patients have an autoimmune disease as well, so the results of the trial could have implications for many patients, said Elad Sharon, M.D., M.P.H., of NCI’s Cancer Therapy Evaluation Program and another leader of the study.
“Releasing the Brakes” on the Immune System
Autoimmune diseases occur when the immune system goes awry and starts to attack the body’s healthy tissues.
Immunotherapy drugs, such as immune checkpoint inhibitors, “release the brakes” on the immune system, allowing immune cells to detect and attack tumor cells.
In some patients, immunotherapy can cause the immune system to recognize some of the body’s healthy tissues as foreign and attack them. This can lead to side effects such as inflammation of the inner lining of the colon, the lungs, or heart muscle.
Some doctors have been concerned that stimulating the immune system against tumors might “unleash the wrath of autoimmunity,” causing potentially severe and even life-threatening complications, Dr. Tawbi noted.
To address such concerns, the new clinical trial will enroll 260 people who have advanced cancer and an autoimmune disease, including dermatomyositis, systemic sclerosis, rheumatoid arthritis, lupus, inflammatory bowel disease, Crohn’s disease, multiple sclerosis, and Sjogren’s syndrome.
Participants will receive nivolumab (Opdivo), an immune checkpoint inhibitor approved by the Food and Drug Administration to treat a number of cancers, including Hodgkin lymphoma and liver, lung, kidney, and bladder cancers.
To determine the safety of the drug in the new trial, the researchers will monitor participants for the sudden emergence of severe autoimmune symptoms, known as flares. The researchers will assess the drug’s effectiveness by tracking patients’ responses to treatment, how long patients survive without their diseases getting worse, and how long patients survive.
The Expanding Use of Immunotherapy
In recent years, the use of immunotherapy for patients with cancer has been expanding rapidly. Immune checkpoint inhibitors, for example, have been tested in a growing number of cancer types, in combination with other therapies, and in patients with different stages of disease.
These efforts underscore the need to develop scientific evidence to guide decisions about whether to use a rapidly growing class of treatments for patients with autoimmune disease and cancer, according to Dr. Sharon.
“One of the main reasons for doing the trial is that immunotherapy has cured some patients with metastatic cancer,” said Dr. Tawbi.
“If we can learn how to use immunotherapy to treat people with both cancer and autoimmune diseases, then we could offer these patients potentially curative therapy,” he added.
Anecdotal Evidence from Treating Patients
Although no clinical trial has tested immunotherapy in cancer patients who have an autoimmune disease, some oncologists have shared their experiences using immunotherapy to treat such patients.
For example, a series of case reports in medical journals has suggested that patients with cancer and an autoimmune disease may respond to immunotherapy drugs as well as patients without autoimmune disease.
“When you activate the immune system, patients may experience more immune-related side effects, as one would expect,” said Alexandra Drakaki, M.D., Ph.D., of the David Geffen School of Medicine at UCLA. But patients who have these side effects might still respond to immune checkpoint inhibitors, she continued.
“We hope to learn more about this from the new NCI-sponsored clinical trial,” she added.
Dr. Drakaki has used immunotherapy drugs to treat some of her patients with advanced cancer and autoimmune disease outside of a clinical trial, and most of the immune-related side effects have been manageable, she said.
In a recent article about treating patients with cancer and an autoimmune disease, Dr. Drakaki and her colleagues wrote that the potential risk of dying from cancer may outweigh the potential harms of worsening autoimmune symptoms, which may be largely reversible.
It may be appropriate to include patients with advanced cancer and autoimmune diseases in clinical trials of checkpoint inhibitors when no other effective options for treating cancer are available, the authors noted.
In her own clinic, some of Dr. Drakaki’s patients with advanced cancer and autoimmune disease have been willing to accept the risk of worsening autoimmune symptoms in exchange for a promising cancer treatment. These patients, she noted, were relatively healthy and had autoimmune diseases that were “well controlled.”
“They just want to be alive,” she said. “They don’t care if their autoimmune symptoms are going to get worse temporarily.”
Dr. Drakaki, however, stressed the need to develop a multidisciplinary care team that can manage cancer as well as autoimmune diseases in patients.
“As oncologists using immunotherapy drugs in patients with autoimmune conditions, we need to work closely with our colleagues who have expertise in treating and managing immune-related side effects,” she said. “We oncologists could not—and should not—do this alone.”
Teams of Experts
The idea of bringing together experts on autoimmune diseases and cancer was central to the development of the new trial.
“We have engaged some of the top experts on autoimmune diseases in the country to provide guidance on classifying the severity of these disorders in patients who also have cancer,” Dr. Tawbi said.
For each type of autoimmune disease that patients in the trial may have, a team of experts has developed treatment strategies and ways to closely monitor the severity of autoimmune symptoms throughout the study.
By developing standard criteria for classifying the severity of autoimmune symptoms and responses to treatment, the researchers hope to create a base of scientific evidence that could be used in the future to select patients with both cancer and an autoimmune disease who are candidates for immunotherapy.
In addition to collecting clinical data, the researchers will collect blood and tissue samples for further investigations, including tissue from organs that had been affected by both autoimmune disease and cancer.
Ultimately, the researchers hope to better understand the mechanisms that give rise to treatment-related side effects in patients with autoimmune diseases, which could provide insights into the biology of autoimmune diseases as well as the side effects of immunotherapy.
“We will study the interactions between the antitumor immune response and the autoimmune response,” said Dr. Tawbi. “This will give us an opportunity to investigate a range of issues, including the dynamics of a flare.”
An exciting aspect of developing the study has been the opportunity to work closely with experts on autoimmune diseases from some of the leading academic medical centers across the United States, noted Dr. Sharon, adding that he expects the collaboration will yield a wealth of information.
“We hope to gain a better understanding of how the immune system changes in patients with autoimmune diseases and cancer who receive immune-directed therapies,” he said. “What we learn could eventually lead to new therapies for patients with autoimmune conditions as well as patients with cancer.”
































No hay comentarios:
Publicar un comentario