
| Clinical Trials | ||
| Updates from the National Cancer Institute | ||
| Clinical Trials News | ||
 | Maintenance Therapy with CC-486 Extends Survival of Adults with AML Maintenance therapy with the drug CC-486 extended overall survival of adults with acute myeloid leukemia (AML) in a large clinical trial. CC-486 is a pill form of the cancer drug azacitidine (Vidaza), which must be administered as an injection at a doctor’s office or treatment center. | |
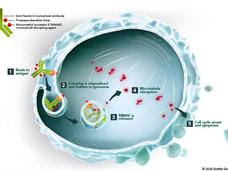 | Enfortumab Vedotin Approved for Recurrent Bladder Cancer The Food and Drug Administration (FDA) has granted accelerated approval for enfortumab vedotin-ejfv (Padcev) to treat people with advanced bladder cancer. The approval was based on response rate and safety results from a subgroup of patients participating in a multi-arm phase 2 trial. | |
| For Metastatic HER2-Positive Breast Cancer, New Treatments Emerge Two new treatment options are emerging for women with metastatic breast cancer, following positive results from clinical trials. The trials tested the drugs tucatinib and trastuzumab deruxtecan (Enhertu) in women who had been previously treated for metastatic HER2-positive breast cancer. | ||
| Clinical Trials Information for Patients and Caregivers | ||
| What Are Clinical Trial Endpoints? In clinical trials, an endpoint is an event or outcome that can be measured objectively to determine whether the intervention being studied is beneficial. The endpoints of a clinical trial are usually included in the study objectives. To learn more about clinical trial endpoints, check out these terms from the NCI Dictionary of Cancer Terms. | ||
 | Find NCI-Supported Clinical Trials Use our search form to find a clinical trial or other research study that may be right for you or a loved one. | |
| NCI-Supported Clinical Trials That Are Recruiting Patients | ||
| Antiviral Therapy for Cancer Patients with History of Hepatitis B This phase 3 trial is testing how well tenofovir alafenamide (Vemlidy) works in preventing liver complications in patients with current or past hepatitis B virus (HBV) who are receiving anticancer therapy for solid tumors. People with chronic or past HBV who are undergoing therapy for cancer are at an increased risk for changes in the liver, which could be minor or severe. Tenofovir alafenamide is a drug that acts against infections caused by HBV. | ||
| Comparing Immunotherapy Drugs for Newly Diagnosed Hodgkin Lymphoma This phase 3 trial is comparing the immunotherapy drugs nivolumab (Opdivo) and brentuximab vedotin (Adcetris) combined with chemotherapy for patients with newly diagnosed stage 3 or stage 4 classic Hodgkin lymphoma. The study will help determine if the addition of one immunotherapy drug to standard chemotherapy improves progression-free survival better than the other. Doctors will also compare the overall survival, event-free survival, and safety of the regimens. | ||
| Immunotherapy Added to Chemoradiotherapy for Bladder Cancer This phase 3 trial will test how well chemotherapy and radiation therapy work with or without the immunotherapy drug atezolizumab (Tecentriq) for treating patients with localized muscle-invasive bladder cancer. Doctors want to see if adding atezolizumab to chemoradiotherapy improves the bladder preservation, overall survival, and duration of complete responses. | ||
































No hay comentarios:
Publicar un comentario