
Adipose Expandability Hypothesis
Adipose tissue is connective tissue primarily comprised of white and brown adipocytes (fat cells). Though previously thought of as an inert tissue, adipocytes have been shown to play varied roles not only in lipid storage but immune, endocrine and nervous function.
The social opinion associated with obesity and fat has changed over centuries. Once considered a sign a wealth and status, modern societies typically view fat with a disapproving eye.
Even though obesity and fat have negative connotations in contemporary society, the numbers of obese individuals has vastly increased globally, with an estimated 15% of the world’s population falling into this category. Between 1975 and 2016 the number of obese individuals has tripled.
In mammals, lipids are primarily stored in adipocytes. They have evolved to provide a safe region for storage within the body, however, the storage sites may reach the upper limit of their capacity when saturated leading to ectopic lipid accumulation in areas such as the liver and muscles. This has the potential to result in a variety of pathological conditions including insulin resistance, dyslipidemia and hypertension.
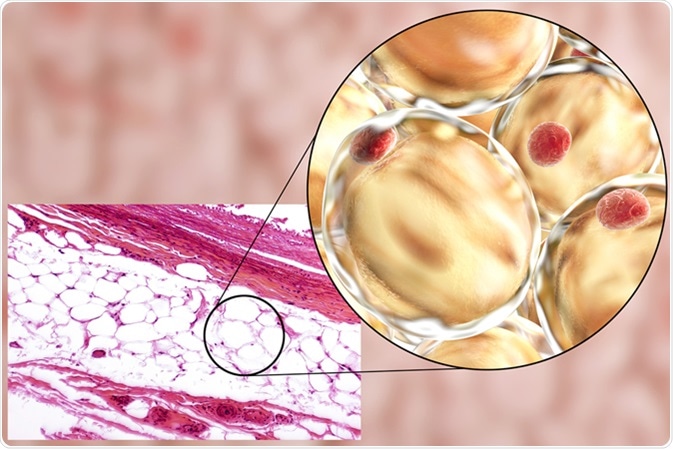
White adipose tissue, light micrograph and 3D illustration, hematoxilin and eosin staining, magnification 100x. Fat cells (adipocytes) have large lipid droplet which remains unstained. Image Credit: Kateryna Kon / Shutterstock
Variation in Adipocytes
Adipocytes can either be defined as brown or white in mammals. White adipocytes (WAT) make up the majority of adipocytes found in the body and are designed for energy storage whereas brown adipocytes (BAT) produce thermal energy.
There are a multitude of mitochondria found within BAT. These mitochondria contain UCP1, a protein which suppresses the proton motive force required to produce Adenosine Tri-Phosphate (ATP) and gives rise to the production of heat.
WAT is the safest place to store lipids, however its storage capacity may become exhausted causing an excess of fat to overspill into non-adipose tissues. This can occur in pathological conditions such as obesity.
WAT not only plays a role in fat storage, it has a plethora of functional roles within the human body. It consists of a variety of cells including fibroblasts, adipocyte precursors and immune cells. WAT can be sub-categorized into two groups, visceral and subcutaneous.
These groups have been observed to have different biological functions, structural forms and molecular signatures. While visceral WAT is generally uniform, subcutaneous WAT is heterogeneous in nature.
The lipid accumulation patterns in these visceral regions may either have protective effects (pear-shaped or female pattern) or lead to the previously mentioned pathological conditions (apple or male pattern).
The hormone, leptin, is known for its ability to suppress food intake and regulate energy balance expressed in WAT . A variety of cytokines (such as TNFα and interleukin 6), fatty acids and growth factors (IGF1 and TGFβ) are also produced within WAT.
Expandability of Adipose Tissue
Every year, there is an approximate 8.4% turnover of adipocytes in humans. An increased calorific intake, results in the proliferation and differentiation of adipocytes. This form of adipogenesis involves the formation of new, small adipocytes which are thought to have protective effects against metabolic dysregulation. Adipose tissue expansion is a continuous process throughout an individual’s lifetime, as adipocytes have a finite lifespan. However, aging is linked an increased body fat percentage.
Adipose stem cells may enable adipose tissue to regenerate. It has been observed in the removal of fat pads in those who have had liposuction or lipectomy often have repeat surgeries. It has also been noted that rats are able to regenerate adipose tissue after 13 weeks indicating that adipose stem cells play an integral role in this process.
In response to stimuli from the environment or homeostasis, adipose tissue expansion may occur. Adipose tissue has the potential to dramatically expand from 3% to 60-70% of the body weight in response to positive energy balances. It can be hypertrophic or hyperplastic in nature. In a hypertrophic response, an individual adipocyte can expand two to threefold. An enhancement in triglyceride storage also occurs. Whereas, in a hyperplastic response, the number of adipocytes increases.
The type of tissue expansion can have an effect on insulin sensitivity; an extended period of hypertrophic expansion is associated with an increase in metabolic dysfunction leading to insulin resistance whereas hyperplastic expansion is thought to be linked to the maintenance of normal insulin sensitivity.
Hypertrophic expansion enables apoptosis (cell death) in adipocytes by promoting local inflammation. It is also able to promote increases in extracellular signaling in molecules like TNFα (which is able to alter the endocrine function of adipose tissues) and interleukin 1 (known to mediate inflammatory responses in adipose tissues) which have negative effects.
Expandability Hypothesis for Future Investigation
The adipose expandability hypothesis presents a logical explanation not only for the link between obesity and metabolic dysfunction but the opportunity to develop better treatment options for obese patients. Further investigation could help in developing a clear picture of the implications of adipose expandability as well as the complexity of both white and brown adipocyte cell lineages.
Sources
- Adipose tissue expandability and the metabolic syndrome https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2474894/
- The developmental origins of adipose tissue https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3775412/
- Brown Fat in Humans: Turning up the Heat on Obesity https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2699856/#B2
- WAT is a functional adipocyte? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3661122/
- Obesity and overweight - World Health Organization http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- TNF-α and adipocyte biology https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4304634/
- IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4553099/
Last Updated: Feb 26, 2019

































No hay comentarios:
Publicar un comentario