P43 Synaptic dysfunctions underlying reduced working memory serial bias in autoimmune encephalitis and schizophrenia
Heike Stein1, Joao Barbosa1, Adrià Galán1, Alba Morato2, Laia Prades2, Mireia Rosa3, Eugenia Martínez4, Helena Ariño4, Josep Dalmau4, Albert Compte3
1Institut d’Investigacions Biomèdiques August Pi i Sunyer (2IDIBAPS), Theoretical Neurobiology, Barcelona, Spain; 2IDIBAPS, Neuroscience, Barcelona, Spain; 3Hospital Clinic, Pediatric Psychiatry, Barcelona, Spain; 4IDIBAPS, Neuroimmunology, Barcelona, Spain
Correspondence: Heike Stein (heike.c.stein@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P43
Continuity of mnemonic contents in time contributes to forming coherent memory representations. Recently, attractive response biases towards previously memorized features in delayed-response tasks have been reported as evidence for the continuous integration of working memory (WM) contents between trials [1]. In turn, brain disorders with reported executive and memory dysfunction may be characterized by reduced WM serial bias [2], revealing reduced temporal coherence of memory representations. To gain mechanistic insight into this effect, we tested a unique population of patients recovering from anti-NMDAR encephalitis patients, an immune-mediated brain disease causing a drastic reduction of NMDARs, accompanied by WM deficits even as receptors return to normal levels [3]. We hypothesized that potential changes in serial biases found in anti-NMDAR encephalitis should be qualitatively similar to changes in schizophrenia, a disorder associated with hypofunctional NMDARs. We collected behavioral data from anti-NMDAR encephalitis patients, schizophrenic patients, and healthy controls performing a visuospatial WM task. While healthy controls’ responses were significantly biased towards previously remembered locations in the presence of WM requirements (delays of several seconds), attractive serial biases were reduced in encephalitis, and absent in schizophrenic patients. We modeled these findings using a recurrent spiking network with synaptic short-term facilitation in excitatory connections. In this model, memory-sustaining bumps of persistent activity decay after the memory delay but leave stimulus-specific, facilitated synaptic ‘traces’ that affect neural dynamics in the next trial. We systematically explored parameters of synaptic transmission and short-term plasticity to determine the mechanism that could reduce attractive serial bias. By altering the parameters of short-term facilitation, we reproduced reduced and absent attractive biases in patient groups, while maintaining WM precision at a constant level across groups, an intriguing finding from our behavioral analyses. This manipulation of short-term facilitation is in accordance with studies in cortical slices from mouse models of schizophrenia [4]. We thus propose that serial biases in visuospatial WM provide a behavioral readout of short-term facilitation dysfunction in anti-NMDAR encephalitis and schizophrenia.
Acknowledgements: Funding provided by Institute Carlos III, Spain (grant PIE 16/00014), Cellex Foundation, the Spanish Ministry of Science, Innovation and Universities (grant BFU 2015-65318-R), the European Regional Development Fund, the Generalitat de Catalunya (grant AGAUR 2017 SGR 1565), “la Caixa” (LCF/BQ/IN17/11620008, H.S.), and the European Union’s Horizon 2020 Marie Skłodowska-Curie grant (713673, H.S.).
References
- 1.Fischer J, Whitney D. Serial dependence in visual perception. Nature Neuroscience 2014, 17, 738–743
- 2.Lieder I, Adam V, Frenkel O, et al. Perceptual bias reveals slow-updating in autism and fast-forgetting in dyslexia. Nature Neuroscience 2019, 22, 256–264
- 3.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurology 2011, 10, 63–74
- 4.Arguello P, Gogos J. Genetic and cognitive windows into circuit mechanisms of psychiatric disease. Trends in Neuroscience 2012, 35, 3–13
P44 Effects of heterogeneity in neuronal electric properties on the intrinsic dynamics of cortical networks
Svetlana Gladycheva1, David Boothe2, Alfred Yu2, Kelvin Oie2, Athena Claudio1, Bailey Conrad1
1Towson University, Department of Physics, Astronomy and Geosciences, Towson, MD, United States of America; 2U.S. Army Research Laboratory, Human Research and Engineering Directorate, Aberdeen Proving Ground, MD, United States of America
Correspondence: Svetlana Gladycheva (sgladycheva@towson.edu)
BMC Neuroscience 2019, 20(Suppl 1):P44
In previous large-scale models of neural systems, neurons of the same class are typically identical. By contrast, real systems exhibit significant cell-to-cell diversity at different levels, from morphology to intrinsic cell properties [1] to synaptic properties [2]. This heterogeneity may affect neural information processing by, for example, helping to integrate diverse inputs to the network [1], or by positively contributing to the stability of the network activity [3]. However, the exact role of neural heterogeneity in large-scale neural systems is not fully understood.
We examine the impact of neural heterogeneity in large-scale neural models. We use an adaptation of the Traub’s single-column thalamocortical network model [4], adapted to the PGENESIS parallel simulation environment [5]. The model is tuned to eliminate intrinsic neuronal activity and is randomly driven with independent Poisson-distributed excitatory postsynaptic noise potentials with an average firing rate between 1-10 Hz.
Network activity is assessed by calculating the mean local field potential (LFP) and analyzing the neuronal spiking activity. We explored changes in network parameters, including local connectivity probability; the parameters of the noise inputs; and the relative strength of synaptic weights. Observed LFPs can generally be classified into two patterns: an aperiodic low-activity state and a high-activity state involving persistent oscillations associated with periodic neuronal firing. At a broad range of the connectivity probabilities, the network stays in low-activity state until a “threshold” level of connectivity is reached. Further increase in connectivity moves model behavior into high-activity regimes and alters the frequency spectrum. Changes in parameters of noise inputs (frequency range, weight, and percentage of neurons receiving noise) elicit similar threshold-like behavior, as do changes in the ratio of excitatory-to-inhibitory synaptic weights, with high-activity states observed in networks with weak inhibition. We introduce heterogeneity in the intrinsic biophysical parameters by randomizing the values of the anomalous rectifier (AR) channels’ conductance in the model’s pyramidal neurons. Preliminary results from effects of heterogeneity on network activity will be shown. In addition, network responses to pulse train stimuli input to the pyramidal cells at different locations in the column will be studied.
References
- 1.Adams NE, et al. Heterogeneity in neuronal intrinsic properties: a possible mechanism for hub-like properties of the rat anterior cingulate cortex during network activity. eNeuro 2017, 0313–16
- 2.Thomson AM, et al, Single axon IPSPs elicited in pyramidal cells by three classes of interneurons in slices of rat neocortex. Journal of Physiology 1996, 496:81–102
- 3.Mejias JF, Longtin A, Differential effects of excitatory and inhibitory heterogeneity on the gain and asynchronous state of sparse cortical networks. Frontiers in Computational Neuroscience 2014, 8:107
- 4.Traub RD, et al, Single column thalamocortical network model exhibiting gamma oscillations, sleep spindles and epileptic bursts. Journal of Neurophysiology 2005, 93(4):2194–232
- 5.Boothe, et al, Impact of neuronal membrane damage on a local field potential in a large-scale simulation of the neuronal cortex. Frontiers in Neurology 2017, 8:236
P45 Structure–function multi-scale connectomics reveals a major role of the fronto-striato-thalamic circuit in brain aging
Paolo Bonifazi1, Asier Erramuzpe1, Ibai Diez1, Iñigo Gabilondo1, Matthieu Boisgontier2, Lisa Pauwels2, Sebastiano Stramaglia3, Stephan Swinnen2, Jesus Cortes1
1Biocruces Health Research Institute, Computational Neuroimaging, Barakaldo, Spain; 2Katholieke Universiteit Leuven, Department of Movement Sciences, Leuven, Belgium; 3University of Bari, Physics, Bari, Italy
Correspondence: Paolo Bonifazi (paol.bonifazi@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P45
Physiological aging affects brain structure and function impacting morphology, connectivity, and performance. However, whether some brain connectivity metrics might reflect the age of an individual is still unclear. Here, we collected brain images from healthy participants (N = 155) ranging from 10 to 80 years to build functional (resting state) and structural (tractography) connectivity matrices, both data sets combined to obtain different connectivity features. We then calculated the brain connectome age—an age estimator resulting from a multi-scale methodology applied to the structure–function connectome, and compared it to the chronological age (ChA). Our results were twofold. First, we found that aging widely affects the connectivity of multiple structures, such as anterior cingulate and medial prefrontal cortices, basal ganglia, thalamus, insula, cingulum, hippocampus, parahippocampus, occipital cortex, fusiform, precuneus, and temporal pole. Second, we found that the connectivity between basal ganglia and thalamus to frontal areas, also known as the fronto-striato-thalamic (FST) circuit, makes the major contribution to age estimation. In conclusion, our results highlight the key role played by the FST circuit in the process of healthy aging. Notably, the same methodology can be generally applied to identify the structural–functional connectivity patterns correlating to other biomarkers than ChA.
P46 Studying evoked potentials in large cortical networks with PGENESIS 2.4
David Beeman1, Alfred Yu2, Joshua Crone3
1University of Colorado, Department of Electrical, Computer and Energy Engineering, Boulder, CO, United States of America; 2U.S. Army Research Laboratory, Human Research and Engineering Directorate, Aberdeen Proving Ground, MD, MD, United States of America; 3U.S. Army Research Laboratory, Computational and Information Sciences Directorate, Aberdeen Proving Ground, MD, MD, United States of America
Correspondence: David Beeman (dbeeman@colorado.edu)
BMC Neuroscience 2019, 20(Suppl 1):P46
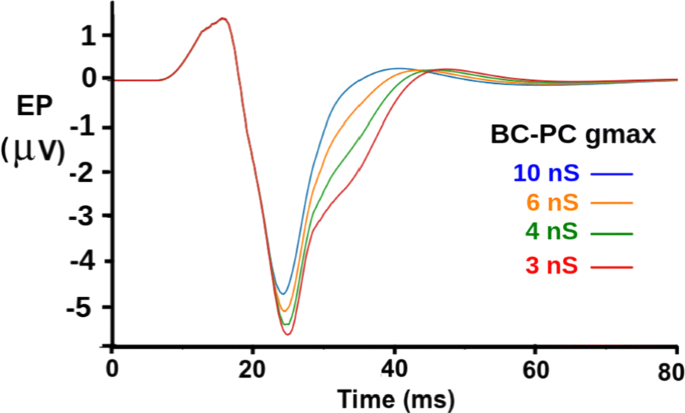
Modern neural simulators have been developed for large scale network models of single-compartment integrate-and-fire neurons that efficiently model millions of neurons. However, accurate modeling of neural activity, including evoked potentials (EPs) recorded from scalp or cortical surface electrodes, requires multicompartmental neuron models with enough realism in the dendritic morphology and location of synapses to account for the major sinks and sources of currents in the extracellular medium. The GENESIS simulator (http://genesis-sim.org) and its parallel computer version PGENESIS were developed over 30 years ago for structurally realistic modeling of large cortical networks. Today, GENESIS continues to be updated with new features and used for implementing such models. Recently Kudela, et al. [1] used a large GENESIS network model to study effects of short-term synaptic plasticity on adaptation of EPs in auditory cortex. Our plans are to increase the size and cell density, extend the model to other cortical layers, and to run simulations on supercomputers such as those available through NSG (the Neuroscience Gateway portal, https://www.nsgportal.org) [2]. Crone, et al. [3] have modified the 2006 release of GENESIS and PGENESIS 2.3 to allow simulations of networks of up to 9 million neurons. Their modifications addressed memory management, reproducibility, and other issues that limited model scalability on high performance computing resources. These improvements are now merged with the current GENESIS/PGENESIS 2.4 development versions. This official release of PGENESIS 2.4 and GENESIS 2.4 are available from the Repository for Continued Development of the GENESIS 2.4 Neural Simulator (https://github.com/genesis-sim/genesis-2.4). We used the new PGENESIS to simulate EPs measured 2 mm above a patch of layer 2/3 primary auditory cortex (Fig. 1), as in [1]. The network was divided into 24 slices simulated in parallel. This model uses 17-compartment pyramidal cells (PCs) based on human cortical PC reconstructions. Inhibition is provided from model basket cells (BCs). Short tone pulses produce excitation to PC distal basal dendrites. Subsequently, PC-PC excitation occurs at oblique apical dendrites. It was shown in [1] that these two excitatory currents produce oppositely oriented electric dipolar charges that are responsible for the initial vertex-positive P1 peak and the following vertex-negative N1 peak in the EP. These results show the effect of varying the strength of the inhibition at the PC proximal apical dendrite from BCs. This occurs later in the N1 peak, and produces a dipole that is oriented oppositely to the one that causes the N1 peak. Therefore, increased inhibition narrows the peak. With PGENESIS available on NSG and other supercomputer resources, we can foster collaborations for using realistic network models to understand human cortical activity.
References
- 1.Kudela P, Boatman-Reich D, Beeman D and Anderson WS. Modeling Neural Adaptation in Auditory Cortex. Front. Neural Circuits 2018, 05 Sept. https://doi.org/10.3389/fncir.2018.00072.
- 2.Sivagnanam S, Majumdar A, Yoshimoto K, Astakhov V, Bandrowski A, Martone ME, Carnevale NT. Introducing the Neuroscience Gateway. IWSG, volume 993 of CEUR Workshop Proceedings 2013 CEUR-WS.org.
- 3.Crone J, Boothe D, Yu A, Olie K, Franaszczuk P. Time step sensitivity in large scale compartmental models of the neocortex. BMC Neurosci 2018 19(Suppl 2):P184.
P47 Automated assessment and comparison of cortical neuron models
Justas Birgiolas1, Russell Jarvis1, Vergil Haynes2, Richard Gerkin1, Sharon Crook2
1Arizona State University, School of Life Sciences, Tempe, United States of America; 2Arizona State University, School of Mathematical and Statistical Sciences, Tempe, AZ, United States of America
Correspondence: Sharon Crook (sharon.crook@asu.edu)
BMC Neuroscience 2019, 20(Suppl 1):P47
Computational models are an indispensable tool for understanding the nervous system. However, describing, sharing, and re-using models with diverse components at many scales represents a major challenge in neuroscience. We have contributed to the development of the NeuroML model description standard [2] and the model sharing platform NeuroML-DB [1] to promote reproducibility and the re-use of data driven neuroscience models. We also have developed the SciDash framework for validating such models against experimental data [4] and sharing the validation outcomes for further scientific discovery at dash.scidash.org, increasing transparency and rigor in the field.
This infrastructure also supports automated pipelines for running large numbers of models shared in the NeuroML format at NeuroML-DB and characterization of these model neurons using simulated “experiments”. These experiments are based on the electrophysiology protocols used by the Allen Cell Type Database [3], which include square, long square, pink noise, ramp, short square and short square triple protocols, and are also the basis for model validation tests. Results are shared in interactive plots at NeuroML-DB. We have characterized over 1000 published cortical neuron models and used the electrophysiological properties of these cortical neuron models to cluster their dynamic behaviors and identify the biophysical properties of models that underlie these clusters. These properties are compared to similar results for experimentally-derived cortical neuron data, providing an overview of how well data-driven models represent the landscape of cortical neuron electrophysiology.
Acknowledgments: This research was funded in part by R01MH106674 from NIMH of the National Institutes of Health and R01EB021711 from NIBIB of the National Institutes of Health.
References
- 1.Birgiolas J, et al. Ontology-assisted keyword search for NeuroML models. In Amarnath Gupta and Susan Rathbun, editors. Proceedings of the 27th International Conference on Scientific and Statistical Database Management 2015. New York, NY: ACM; article 37.
- 2.Gleeson P, et al. NeuroML: a language for describing data driven models of neurons and networks with a high degree of biological detail. PLoS Computational Biology 2010, 6, e1000815.
- 3.Hawrylycz M, et al. Inferring cortical function in the mouse visual system through large-scale systems neuroscience. PNAS 2016, 113(27), 7337–44.
- 4.Omar C, et al. Collaborative infrastructure for test-driven scientific model validation. In Companion Proceedings of the 36th International Conference on Software Engineering 2014 May 31 (pp. 524–527). ACM.
P48 High dimensional ion channel composition enables robust and efficient targeting of realistic regions in the parameter landscape of neuron models
Marius Schneider1, Peter Jedlicka2, Hermann Cuntz3,4
1University of Frankfurt, Institute for Physics, Butzbach, Germany; 2Justus Liebig University, Faculty of Medicine, Giessen, Germany; 3Frankfurt Institute for Advanced Studies (FIAS), Frankfurt am Main, Germany; 4Ernst Strüngmann Institute (ESI), Computational Neuroanatomy, Frankfurt am Main, Germany
Correspondence: Marius Schneider (marius-s@online.de)
BMC Neuroscience 2019, 20(Suppl 1):P48
Cellular and molecular sources of variability in the electrical activity of nerve cells are not fully understood. An improved understanding of this variability is the key to predict the response of nerve tissue to pathological changes. We have previously created a robust data-driven compartmental model of the hippocampal granule cell comprising 16 different ion channels and variable dendritic morphologies. Here, we show that it is possible to drastically reduce ion channel diversity while preserving the characteristic spiking behavior of real granule cells. In order to better understand the variability in spiking activity we generated large populations of validated granule cell models with different numbers of ion channels. Unreduced or less reduced models with a higher number of ion channels covered larger and more widely spread regions of the parameter landscape. Moreover, unreduced or less reduced models with a higher number of ion channels were more stable in the face of parameter perturbations. This suggests that ion channel diversity allows for increased robustness and higher flexibility of finding a solution in the complex parameter space. In addition to increasing our understanding of cell-to-cell variability, our models might be of practical relevance. Instead of a one-size-fits-all approach where a computer model simulates average experimental values, the population-based approach reflects the variability of experimental data and therefore might enable pharmacological studies in silico.
P49 Modelling brain folding using neuronal placement according to connectivity requirements
Moritz Groden1, Marvin Weigand2,3, Jochen Triesch3, Peter Jedlicka4, Hermann Cuntz2,3
1Justus Liebig University Giessen, Faculty of Medicine, Mannheim, Germany; 2Ernst Strüngmann Institute (ESI), Computational Neuroanatomy, Frankfurt am Main, Germany; 3Frankfurt Institute for Advanced Studies (FIAS), Neuroscience, Frankfurt am Main, Germany; 4Institute of Clinical Neuroanatomy Frankfurt, ICAR3R-Justus-Liebig University Giessen, Faculty of Medicine, Giessen, Germany
Correspondence: Moritz Groden (moritzgromail@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P49
Among different species of animals, the layout of the central nervous system varies extensively from individual clusters of neurons (ganglia) in invertebrates such as worms to solid brains found in mammals that typically exhibit increased folding the larger the animal. Such variations in layout may point to elemental differences in organization of circuitry and connectivity. However, many studies suggest that folding of the brain is a consequence of the restricted volume of the skull exerting mechanical forces on the cortex, which in turn folds to fit a larger surface area into such a confined cavity. In our study we consider a computational model that uses dimension reduction methods to ensure optimal placement of neurons, placing them according to connectivity needs, rather than modelling the forces exerted on the cortex. We assume a simple connectivity that features strong local but weak global (long-range) connections, which mimics the connectivity found in mammalian brains. The predictions made by our model cover all different phenotypes of brains found in animals, ranging from individual ganglia through smooth brains with no gyrification, to extremely convoluted brains for increasing cortical size. Many properties of the cortical morphology found in animals are reproduced by the model, which includes metrics such as the folding index and the fractal dimension. Our model presents a way to combine microscopic inter cellular connectivity with macroscopic morphologies into large-scale brain models that feature its neural network requirements.
P50 Dynamic neural field modeling of auditory categorization tasks
Pake Melland1, Bob McMurray2, Rodica Curtu1
1University of Iowa, Department of Mathematics, Iowa City, United States of America; 2University of Iowa, Psychological and Brain Sciences, Iowa City, United States of America
Correspondence: Rodica Curtu (rodica-curtu@uiowa.edu)
BMC Neuroscience 2019, 20(Suppl 1):P50
Categorization is the fundamental ability to treat distinct stimuli similarly; categorization applied to auditory stimuli is crucial for speech perception. For example, phonemes like “t” and “d” are categories that generalize across speakers and contexts. A fundamental question asks what mechanisms form the foundation for auditory category learning. We propose a dynamic neural network framework that combines plausible biological mechanisms and the theory of dynamic neural fields to model this process. The network models a task designed to emulate first language acquisition—a period of unsupervised learning followed by supervised learning. In the unsupervised phase the listener is presented with a sequence of pairs of tones; each pair corresponds to one of four categories defined by their frequencies. During this time the subject engages in a non-distracting task. Then the subject engages in a supervised task and is instructed to associate each tone-pair with a physical object representing one of the four auditory categories. Corrective feedback is given to the subject during the supervised learning. The mathematical model is used to manipulate mechanisms through which hypotheses can be made about the category learning process. We present preliminary results from model simulations of the experiment and compare them with implementations of the experiment on human subjects.
Network Description and Results. We propose a dynamic neural field composed of multiple layers allowing for the manipulation and testing of multiple locations of plasticity involved in the learning process. First, incoming sounds stimulate a one-dimensional tonotopically organized feature space composed of neural units that interact through local excitation with lateral inhibition. Units along this space are associated with sub-cortical auditory fields and respond to specific frequencies in order to capture physical properties of the stimuli. Activity in the feature space feeds forward through excitatory connections, which undergo depression with prolonged stimuli encounters, to regions of primary and secondary auditory cortex. Activity in these regions provide input to the category layer of the network composed of 4 neural units corresponding to the 4 categories defined in the task. These nodes are hypothesized to represent regions in auditory-related temporal cortical regions such as superior temporal gyrus [2] and the inferior frontal gyrus in humans, or the prefrontal cortex in rats [1]. In the theoretical network, the four category nodes are coupled through mutual inhibition and compete in a winner take all setting. Above threshold activation peaks in the category layer are interpreted as experimentally detectable responses. In the supervised portion of the task, synapses between auditory cortex nodes and category layer nodes are updated with via Hebbian processes with a reward/punishment parameter that serves as corrective feedback to the network. Parameters within the model are tuned so that responses in the category layer closely match behavioral results obtained from implementations of the experiment on human subjects that varied stimuli distributions, category prototypes, and category boundaries. The model predicts category learning at rates consistent with those found experimentally.
Acknowledgments: NSF CRCNS 151567.
References
- 1.Francis NA, Winkowski DE, Sheikhattar A, Armengol K, Babadi B, Kanold PO. Small networks encode decision-making in primary auditory cortex. Neuron 2018 Feb 21;97(4):885–97.
- 2.Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science 2014 Feb 28;343(6174):1006–10.
P51 Role of TRP channels in temperature rate coding by drosophila noxious cold sensitive neurons
Natalia Maksymchuk, Akira Sakurai, Atit Patel, Nathaniel Himmel, Daniel Cox, Gennady Cymbalyuk
Georgia State University, Neuroscience Institute, Atlanta, GA, United States of America
Correspondence: Natalia Maksymchuk (nmaksymchuk1@gsu.edu)
BMC Neuroscience 2019, 20(Suppl 1):P51
Noxious cold temperature can cause tissue damage and triggers protective behaviors of animals. Cellular mechanisms of noxious cold temperature coding are not well understood. We focus on Drosophila larval cold nociception capitalizing on a diverse array of approaches spanning genetics and animal behavior to electrophysiology and computational models. Larva responds to noxious cold by a well-characterized full-body contraction. Notably, this response is only triggered by a sufficiently fast temperature change. Class III (CIII) multidendritic sensory neurons and specific TRP channels are implicated in noxious cold temperature coding [1]. Based on Ca2+ imaging, specialized roles of Trpm and Pkd2 currents were established and our model explained an apparent paradox of these data [1, 2].
We performed electrophysiological recordings and Ca2+ imaging of CIII neurons along with behavioral analyses. We compared responses of wild type to slow and fast temperature changes from 24oC down to the 10oC. Cold-evoked contraction behavior was potentiated under fast ramping conditions relative to slow. Spiking and [Ca2+]i response at noxious cold were consistent with behavioral data. The CIII neurons exhibited a pronounced peak of spiking rate when the temperature was rapidly decreased and turned silent as the temperature was increased back to 24oC. The response was different when temperature changed slowly: the spiking rate was much smaller during the temperature decrease.
These results suggest that CIII neurons encode rate of temperature decrease. We hypothesize that inactivation processes of certain TRP channels could explain these differences. We focused on comparison of the roles of Pkd2 and Trpm currents as temperature sensors. Our computational model showed that the Ca2+-dependence of the Pkd2 inactivation constant could provide a mechanism of observed rate coding. This mechanism, implemented in the model, allowed us to reproduce recorded electrical activity data—high peak of firing rate in response to the rapid temperature change from 24oC to 10oC and silence during temperature return back to ambient levels. When the noxious cold temperature was held constant after fast ramp, Pkd2 channels inactivated, and low-frequency firing rate was supported through Trpm, responsible for coding temperature. This is consistent with behavioral data as well. In addition, the model shows that increased firing rate at fast temperature decline was accompanied by high [Ca2+]i level, whereas slow ramp resulted in significantly lower Ca2+. We conclude that certain TRP channels, such as Pkd2, could be responsible for high peak of firing rate at rapid temperature fall, whereas Trpm channels could encode the magnitude of temperature.
Acknowledgements: This work was supported by NIH R01 NS086082 and a GSU Brains & Behavior Seed Grant (DNC). NJH is a Brains and Behavior and Honeycutt Fellow; AAP is a 2CI Neurogenomics and Honeycutt Fellow.
References
- 1.Turner HN, Armengol K, Patel AA, et al. The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila. Current Biology 2016, 26(23), 3116–3128.
- 2.Maksymchuk N, Patel AA, Himmel NJ, Cox DN, Cymbalyuk G. Modeling of TRP channel mediated noxious cold sensation in Drosophila sensory neurons. BMC Neuroscience 2018, 19(Suppl 2):64, 8–9.
P52 Role of Na+/K+ pump in dopamine neuromodulation of a mammalian central pattern generator
Alex Vargas, Gennady Cymbalyuk
Georgia State University, Neuroscience Institute, Atlanta, GA, United States of America
Correspondence: Alex Vargas (avarg453@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P52
CPGs are oscillatory neuronal circuits controlling rhythmic movements across vertebrates and invertebrates [1]. The Na/K pump contributes to the dynamics of bursting activity in variety of CPGs seen across species such as leech, tadpole, and mouse [2, 3, 4, 5, 6]. Movements like locomotion and heartbeat must be continually regulated for an animal to meet environmental and behavioral demands [3]. In vertebrate CPGs, dopamine has been shown to induce a range of subtle to pronounced effects on locomotory and other motor rhythms. Dopamine neuromodulation affects Na/K Pump, GIRK2-, A-, and h-currents through D1 and D2 receptors [7, 8]; this contributes to stabilization of CPG rhythmic activity. We developed a half-center oscillator (HCO) model of a spinal locomotor CPG, which comprises of four populations, two inhibitory and two excitatory. Under a certain parameter regime, the neurons are intrinsically bursting, utilizing a persistent-sodium current mechanism. We investigated activity regimes of single endogenously bursting neurons and HCO. In a range of high modulation level, we found stable periodic bursting, while within some range of low dopamine modulation levels, pronounced intermittent intrinsic patterns. We investigated the hypothesis that dopamine affects the network through activation of inward rectifying potassium currents, IGIRK and IA, and opposing changes of h-current all while interacting with pump current. The reduction in modulatory level of dopamine in the spinal locomotor CPG causes the model to transition from normal periodic bursting into intermittent bursting and then to silence. Our locomotor CPG model highlights the role of the pump and its co-modulation along with GIRK2-, A-, and h-currents in production of robust rhythmic output.
Acknowledgements: Supported by NINDS 1 R21 NS111355 to GC.
References
- 1.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiological reviews 1996 Jul 1;76(3):687–717.
- 2.Picton LD, Zhang H, Sillar KT. Sodium pump regulation of locomotor control circuits. Journal of neurophysiology 2017 May 24;118(2):1070–81.
- 3.Sharples SA, Whelan PJ. Modulation of rhythmic activity in mammalian spinal networks is dependent on excitability state. eNeuro 2017 Jan;4(1).
- 4.Sharples SA, Humphreys JM, Jensen AM, et al. Dopaminergic modulation of locomotor network activity in the neonatal mouse spinal cord. Journal of neurophysiology 2015 Feb 4;113(7):2500–10.
- 5.Kueh D, Barnett WH, Cymbalyuk GS, Calabrese RL. Na+/K+ pump interacts with the h-current to control bursting activity in central pattern generator neurons of leeches. eLife 2016 Sep 2;5:e19322.
- 6.Tobin AE, Calabrese RL. Myomodulin increases I h and inhibits the Na/K pump to modulate bursting in leech heart interneurons. Journal of neurophysiology 2005 Dec;94(6):3938–50.
- 7.Sharples SA, Whelan PJ. Modulation of rhythmic activity in mammalian spinal networks is dependent on excitability state. eNeuro 2017 Jan;4(1).
- 8.Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. Journal of Neuroscience 2007 Nov 28;27(48):13192–204.
P53 Hypoxic suppression of Ca2+-ATPase pumps and mitochondrial membrane potential eliminates rhythmic activity of simulated interstitial cells of Cajal
Sergiy Korogod1, Iryna Kulagina1, Parker Ellingson2, Taylor Kahl2, Gennady Cymbalyuk2
1Bogomoletz Institute of Physiology, National Academy of Sciences of Ukraine, Kiev, Ukraine; 2Georgia State University, Neuroscience Institute, Atlanta, GA, United States of America
Correspondence: Gennady Cymbalyuk (gcymbalyuk@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P53
Neonatal hypoxic-ischemic injury is a risk factor for necrotizing enterocolitis (NEC), an inflammatory bowel disease that is often associated with failures of gastrointestinal motility. This motility is driven by a pacemaker action of the interstitial cells of Cajal (ICCs) on intestinal smooth muscle cells (SMCs). The ICC pacemaker activity is determined by interplay of Ca2+channels, pumps, and exchangers present in the endoplasmic reticulum (ER), mitochondria and plasma membrane to form a characteristic Ca2+-handling mechanism. Ca2+-ATPase pumps in ICC are potential targets for injuring action of hypoxia as they operate by consuming energy stored in ATP due to oxidative phosphorylation in mitochondria. In an ICC model, we mimicked effects of hypoxia by reduction of the mitochondrial bulk membrane potential (ΔΨ*) or maximal rates of Ca2+-ATPase pumps in the plasmalemma or ER (PMCA or SERCA, respectively). ICC pacemaker activity (oscillations of the plasma membrane potential Emand intracellular calcium concentration [Ca2+]i) ceased by individual suppression of ΔΨ*, or PMCA, or SERCA and the cessation scenarios were case-specific. Since naturally hypoxia simultaneously affects all these actors, in this study, we explored scenarios of cessation of ICC pacemaker activity depending on combined suppression of ΔΨ*, PMCA, and SERCA. At fixed normal ΔΨ*, equal joint suppression of PMCA and SERCA dramatically reduced amplitude of [Ca2+]iand Emoscillations to “downstate” levels near their basal/rest values. This was similar to the effect of individual suppression of SERCA and dissimilar to that of PMCA, which was characterized by very low-amplitude oscillations about “upstate” levels of depolarized Emand elevated [Ca2+]i. In each case, changes in oscillations frequency were insignificant. Same suppression of PMCA and SERCA accompanied by that of ΔΨ*ceased the ICC pacemaker activity according to scenario observed during isolated reduction of ΔΨ*: the oscillations frequency reduced, duration of oscillatory plateaus of Emand [Ca2+]iextended and, at certain critically low ΔΨ*, the oscillations totally ceased and “downstate” basal [Ca2+]iand rest Emwere established.
Hence, hypoxic suppression of the above considered energy-producing and energy-consuming mechanisms in any combination led the cessation of ICC pacemaker activity and establishment of [Ca2+]iand Em“downstates” near their basal/rest levels without any or with very small oscillations. For the cessation scenario, the main governing factor was suppression of ΔΨ*, and among the Ca2+-ATPase pumps SERCA dominated over PMCA. The observed effects may have crucial pathological consequences for ICC-driven periodic contractions of electrically coupled SMCs manifested as gastrointestinal dysmotility and development of NEC. Since similar Ca2+-handling mechanisms operate in other type excitable cells, particularly in neurons, our model and protocols of computational experiments can be adapted for simulation studies of cellular mechanisms functional consequences of hypoxic injuries of the brain and spinal cord.
P54 Reconstruction and simulation of the cerebellar microcircuit: a scaffold strategy to embed different levels of neuronal details
Claudia Casellato1, Alice Geminiani2, Alessandra Pedrocchi2, Elisa Marenzi1, Stefano Casali1, Chaitanya Medini1, Egidio D’Angelo1
1University of Pavia, Dept. of Brain and Behavioral Sciences - Unit of Neurophysiology, Pavia, Italy; 2Politecnico di Milano, Department of Electronics, Information and Bioengineering, Milan, Italy
Correspondence: Claudia Casellato (claudia.casellato@unipv.it)
BMC Neuroscience 2019, 20(Suppl 1):P54
Computational models allow propagating microscopic phenomena into large-scale networks and inferencing causal relationships across scales. Here we reconstruct the cerebellar circuit by bottom-up modeling, reproducing the peculiar properties of this structure, which shows a quasi-crystalline geometrical organization well defined by convergence/divergence ratios of neuronal connections and by the anisotropic 3D orientation of dendritic and axonal processes [1].
Therefore, a cerebellum scaffold model has been developed and tested. It maintains scalability and can be flexibly handled to incorporate neuronal properties on multiple scales of complexity. The cerebellar scaffold includes the canonical neuron types: Granular cell, Golgi cell, Purkinje cell, Stellate and Basket cells, Deep Cerebellar Nuclei cell. Placement was based on density and encumbrance values, connectivity on specific geometry of dendritic and axonal fields, and on distance-based probability.
In the first release, spiking point-neuron models based on Integrate & Fire dynamics with exponential synapses were used. The network was run in the neural simulator pyNEST. Complex spatiotemporal patterns of activity, similar to those observed in vivo, emerged [2].
For a second release of the microcircuit model, an extension of the generalized Leaky Integrate & Fire model has been developed, optimized for each cerebellar neuron type and inserted into the built scaffold [3]. It could reproduce a rich variety of electroresponsive patterns with a single set of optimal parameters.
Complex single neuron dynamics and local connectome are key elements for cerebellar functioning.
Then, point-neurons have been replaced by detailed 3D multi-compartment neuron models. The network was run in the neural simulator pyNEURON. Further properties emerged, strictly linked to the morphology and the specific properties of each compartment.
This multiscale tool with different levels of realism has the potential to summarize in a comprehensive way the electrophysiological intrinsic neural properties that drive network dynamics and high-level behaviors.
The model, equipped with ad-hoc plasticity rules, has been embedded in a sensorimotor loop of EyeBlink Classical Conditioning. The network output evolved along repetitions of the task, therefore letting emerge three fundamental operations ascribed to the cerebellum: prediction, timing and learning of motor commands.
Acknowledgments: This research was supported by the HBP Neuroinformatics, Brain Simulation, and HPAC Platforms, funded by European Union’s Horizon 2020 under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2), also involving the HBP Partnering Project CerebNEST.
References
- 1.D’Angelo E, Antonietti A, Casali S, et al. Modeling the cerebellar microcircuit: new strategies for a long-standing issue. Frontiers in cellular neuroscience 2016 Jul 8;10:176.
- 2.Casali S, Marenzi E, Medini KC, Casellato C, D‘Angelo E. Reconstruction and Simulation of a Scaffold Model of the Cerebellar Network. Frontiers in Neuroinformatics 2019;13:37.
- 3.Geminiani A, Casellato C, Locatelli F, et al. Complex dynamics in simplified neuronal models: reproducing Golgi cell electroresponsiveness. Frontiers in Neuroinformatics 2018, 12, 1–19; https://doi.org/10.3389/fninf.2018.00088
P55 Simplified and physiologically detailed reconstructions of the cerebellar microcircuit
Elisa Marenzi1, Chaitanya Medini1, Stefano Casali1, Martina Francesca Rizza1, Stefano Masoli1, Claudia Casellato2, Egidio D’Angelo2
1University of Pavia, Department of Brain and Behavioural Sciences, Pavia, Italy; 2University of Pavia, Dept. of Brain and Behavioral Sciences - Unit of Neurophysiology, Pavia, Italy
Correspondence: Elisa Marenzi (elisa.marenzi@unipv.it)
BMC Neuroscience 2019, 20(Suppl 1):P55
The cerebellum is the second largest cortical structure of the brain and contains about half of all brain neurons. Its modeling brings issues reflecting the peculiar properties of the circuit, which has a quasi-crystalline geometrical organization defined by convergence/divergence ratios of neuronal connections and by the anisotropic 3D orientation of dendritic and axonal processes [1]. A data-driven scaffold [2] comprising the granular (GrL), Purkinje (PL), molecular (ML) and Deep Cerebellar Nuclei (DCN) layers has been developed for testing network models with different complexities.
Its reconstruction follows sequential steps. Firstly, cells are placed in the simulation volume through an ad-hoc procedure: the GrL contains glomeruli (glom), granule cells (GrC) and Golgi cells (GoC); somata of Purkinje cells (PC) are in the PL while their dendritic trees are in the ML; here molecular layer interneurons (MLI)—stellate (SC) and basket cells (BC)—are placed whereas the DCN contains only the glutamatergic cells (DCNC).
The connectome stores the IDs of pre- and post-synaptic neurons. Parameters and morphological features derived from physiological experiments and literature data are the basis for its reconstruction, built on geometrical and probability-based rules. When using detailed neuronal morphologies, such rules have been improved to determine dendrites connected also through a touch detection algorithm.
The most typical behaviors of this microcircuit have been tested for both kinds of networks (pyNEST for the point-neuron version and pyNEURON when all detailed morphologies were available). Neuronal discharge of the different neuron populations in response to a mossy fiber burst have been evaluated, showing very similar results between the two simulators. In particular, GoC, SC and BC generate inhibitory bursts that contribute to terminate the GrC and PC bursts and to produce the burst-pause PC response.
Another important behavior regards the PC activation and sensitivity to molecular layer connectivity. The pattern of activity is determined by the various connection properties: particularly, PC inhibition is achieved through a differential orientation between SC and BC axons, while PC excitation depends on both ascending axons (aa) and pf synapses with specific origin from GrC. Their spatial extension reflects the propagation of activity through the MLI network.
The additional details introduced in pyNEURON simulations highlight more complex and physiologically relevant results that cannot be explained with a simplified model without dendrites. Moreover, the integration of the Inferior Olive completes the closed loop of the microcircuit, allowing to embed functional plasticity able to simulate learning processes.
Acknowledgements: The research was supported by the EU Horizon 2020 under the Specific Grant Agreements No. 720270 (HBP SGA1) and 785907 (HBP SGA2).
References
- 1.D’Angelo E, Antonietti A, Casali S, et al. Modeling the cerebellar microcircuit: new strategies for a long-standing issue. Frontiers in cellular neuroscience 2016 Jul 8;10:176.
- 2.Casali S, Marenzi E, Medini KC, Casellato C, D‘Angelo E. Reconstruction and Simulation of a Scaffold Model of the Cerebellar Network. Frontiers in neuroinformatics 2019;13:37.
P56 A richness of cerebellar granule cell discharge properties predicted by computational modeling and confirmed experimentally
Stefano Masoli1, Marialuisa Tognolina1, Francesco Moccia2, Egidio D’Angelo1
1University of Pavia, Department of Brain and Behavioural Sciences, Pavia, Italy; 2University of Pavia, Department of Biology and Biotechnology “L. Spallanzani”, Pavia, Italy
Correspondence: Stefano Masoli (stefano.masoli@unipv.it)
BMC Neuroscience 2019, 20(Suppl 1):P56
The cerebellar granule cells (GrCs) are the most common neuron type in the central nervous system. Their highly packed distribution and misleading simple cytoarchitecture, generated the idea of a limited spike generation mechanism. The regular spikes discharge, recorded for short periods of time (<800ms), was the cornerstone for the simulation of realistic [1, 2]. We show that GrCs are capable of diverse patterns response when subjected to prolonged current inject (2s). The somato-dendritic sections were taken from [3], extend with a single section Hillock, an Axon Initial Segment (AIS), an ascending axon and two thin 1mm long parallel fibers. The ionic channels were taken from [1, 2, 4]. The Nav1.6 sodium channel was improved with FHF14 and located in the Hillock and AIS [5]. The calcium buffer was reworked to contain only Calretinin. The models, were automatically fitted with BluePyOpt/NEURON [6]. After 0.8-1s of regular firing, the models predicted three possible outcomes: 1) regular firing, 2) mild adaptation and 3) strong adaptation of firing. Patch-clamp experimental recordings (current-clamp configuration, parasagittal slices obtained from p18-24 Wistar rats) confirmed the modelling predictions on firing adaptation. In a subset of experiments GrCs showed firing acceleration that was not found by the optimization technique. To simulate these GrCs, a TRPM4 channel, known to mediate slow depolarizing currents, was linked to Calmodulin (Cam2C) concentration. This mechanism allowed to reach the accelerated state. These different firing properties impacted on synaptic excitation when the mossy fiber bundle was stimulated at different frequencies (1-100 Hz). Interestingly, a range of different filtering properties emerged, with some cells showing one-to-one responses while others responding faster or slower than the input. This modelling and experimental effort described GrCs properties that show the richness of their encoding capabilities.
Acknowledgements: This project has received funding from the Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2).
References
- 1.D’Angelo E, Nieus T, Maffei A, et al. Theta-frequency bursting and resonance in cerebellar granule cells: experimental evidence and modeling of a slow k+-dependent mechanism. Journal of Neuroscience 2001;21:759–70.
- 2.Diwakar S, Magistretti J, Goldfarb M, Naldi G, D’Angelo E. Axonal Na+ channels ensure fast spike activation and back-propagation in cerebellar granule cells. Journal of Neurophysiology 2009;101:519–32.
- 3.Masoli S, Rizza MF, Sgritta M, Van Geit W, Schürmann F, D’Angelo E. Single Neuron Optimization as a Basis for Accurate Biophysical Modeling: The Case of Cerebellar Granule Cells. Frontiers in cellular neuroscience 2017;11:1–14.
- 4.Masoli S, Solinas S, D’Angelo E. Action potential processing in a detailed Purkinje cell model reveals a critical role for axonal compartmentalization. Frontiers in cellular neuroscience 2015;9:1–22.
- 5.Dover K, Marra C, Solinas S, et al. FHF-independent conduction of action potentials along the leak-resistant cerebellar granule cell axon. Nature Communications 2016;7:12895.
- 6.Van Geit W, Gevaert M, Chindemi G, et al. BluePyOpt: Leveraging Open Source Software and Cloud Infrastructure to Optimise Model Parameters in Neuroscience. Frontiers in Neuroinformatics 2016;10:1–30.
































No hay comentarios:
Publicar un comentario