P57 Spatial distribution of Golgi cells inhibition and the dynamic geometry of Cerebellum granular layer activity: a computational study
Stefano Casali1, Marialuisa Tognolina1, Elisa Marenzi1, Chaitanya Medini1, Stefano Masoli1, Martina Francesca Rizza1, Claudia Casellato2, Egidio D’Angelo1
1University of Pavia, Department of Brain and Behavioural Sciences, Pavia, Italy; 2University of Pavia, Department of Brain and Behavioural Sciences - Unit of Neurophysiology, Pavia, Italy
Correspondence: Stefano Masoli (stefano.masoli@unipv.it)
BMC Neuroscience 2019, 20(Suppl 1):P57
The cerebellum granular layer (GL) has been considered for a long time as a fine-grained spatio-temporal filter, characterized by its main role of delivering the right amount of information at the proper timing to the above molecular layer (ML) [1] While this general tenet remains, recent experimental and theoretical works suggest that the GL is endowed with a rich and complex variety of spatio-temporal dynamics, empowering the GL itself to exert a qualitatively strong influence upon the nature of the signal conveyed to the ML.
In the present work, a large-scale computational reconstruction of the GL network has been developed, exploiting previously published detailed single cell models of granule cells (GrCs, [2]) and Golgi cells [3]. The peculiar structure of synaptic connections has been observed and reproduced by means of geometrical-statistical connectivity rules derived from experimental data, when available [4]. One of the main features of GL connectivity, the anisotropic organization of GoCs axonal plexus, which is orthogonal to the parallel fibers (pfs, coronal axis) and runs along the parasagittal axis, plays a key role in shaping the spatio-temporal dynamics of GL activity. Excitatory / inhibitory ratio of GrCs response to external stimuli is organized in a center-surround structure, with excitation prevailing in the core and inhibition in the surround area [5] Simulations results show that Golgi cells inhibition is stronger along the parasagittal axis; these computational predictions have been confirmed by a set of experiments in acute slices in vitro with high resolution two-photon microscopy. This preferential path for Golgi cells inhibition can also affect how two simultaneously activated distant spots interact: simulations show that spots placed at a 100 or 200mm distance along the parasagittal axis can significantly inhibit each other; on the contrary, when the spots are positioned along the coronal axis, in line with the pfs, almost no interaction occurs. Specific synapses modulate the strength of this phenomenon; specifically, when the ascending axon (aa) synapses from GrCs to GoCs are switched-off, inhibitory interaction along the parasagittal axis decreases.
Acknowledgements: The research was supported by the EU Horizon 2020 under the Specific Grant Agreements No. 720270 (HBP SGA1) and 785907 (HBP SGA2).
References
- 1.Rössert C, Dean P, Porril J. At the Edge of Chaos: How Cerebellar Granular Layer Network Dynamics Can Provide the Basis for Temporal Filters. PLoS Computational Biology 2015. 11(10). 1–28
- 2.D’Angelo E, Nieus T, Maffei A, et al. Theta-frequency bursting and resonance in cerebellar granule cells: experimental evidence and modeling of a slow K+-dependent mechanism. Journal of Neuroscience 2001. 21. 759–770
- 3.Solinas S, Forti L, Cesana E, et al. Fast-reset of pacemaking and theta-frequency resonance patterns in cerebellar Golgi cells: simulations of their impact in vivo. Frontiers in Cellular Neuroscience 2007. 1. 1–9
- 4.Korbo L, Andresen BB, Ladefoged O, et al. Total numbers of various cell types in rat cerebellar cortex estimated using an unbiased stereological method. Brain Research 1993. 609. 262–268
- 5.Mapelli J, D’Angelo E. The Spatial Organization of Long-Term Synaptic Plasticity at the Input Stage of Cerebellum. Journal of Neuroscience 2007. 27. 1285–1296
P58 Reconstruction and simulation of cerebellum granular layer functional dynamics with detailed mathematical models
Chaitanya Medini1, Elisa Marenzi1, Stefano Casali1, Stefano Masoli1, Claudia Casellato2, Egidio D’Angelo2
1University of Pavia, Department of Brain and Behavioural Sciences, Pavia, Italy; 2University of Pavia, Dept. of Brain and Behavioral Sciences - Unit of Neurophysiology, Pavia, Italy
Correspondence: Elisa Marenzi (elisa.marenzi@unipv.it)
BMC Neuroscience 2019, 20(Suppl 1):P58
Cerebellum has been widely known to be involved in several cognitive activities, however an elaborate investigation is required to validate known hypotheses and propose new theories. A detailed large-scale scaffold cerebellar circuit was developed with experimental connectivity rules on python NEURON with MPI configuration. An adaptable version of the cerebellar scaffold model [1] is developed on pyNEST and pyNEURON using morphologically-driven cell positions and functional connectivity, inspired from convergence/divergence geometry rules [2]. The reconstruction methodologies used for scaffold network improvises on the existing connectivity literature with Bounded Self-Avoiding Random Walk Algorithm. The simulations revealed a close correspondence to experimental results validating the network reconstruction. Simulations in pyNEURON gave results like those obtained with pyNEST. This is an important validation to ensure that the connectivity generates identical functional dynamics irrespective of the simulator platform. In the current study, pyNEURON scaffold cerebellar model has been extended from point neuron model network to detailed biophysical model network with similar connectome and positions. Detailed multicompartmental models of granule [3], Golgi, Purkinje [4], Stellate and Basket neurons (to be published) are being used for the study. As a first test case, the detailed neuron morphologies are connected using simpleneuronal connectivity rules representing spatially confined convergence/divergence rules. The number of synapses were evenly distributed along the dendritic length of these neuron models to compensate for the absence of computed distance probability between pre and post synaptic neurons. In the second case, a touch-detector based algorithm [5], was used to generate synaptic connectivity in the molecular layer (including Molecular Layer Interneurons and Purkinje Neurons). The network implementation is scalable and flexible to include new types of cell models or to replace the current version with updated models.
Acknowledgements: The research was supported by the EU Horizon 2020 under the Specific Grant Agreements No. 720270 (HBP SGA1) and 785907 (HBP SGA2).
References
- 1.Casali S, Marenzi E, Medini KC, Casellato C, D‘Angelo E. Reconstruction and Simulation of a Scaffold Model of the Cerebellar Network. Frontiers in neuroinformatics 2019;13:37. https://doi.org/10.1101/532515
- 2.Solinas S, Nieus T, D‘Angelo E. A realistic large-scale model of the cerebellum granular layer predicts circuit spatio-temporal filtering properties. Frontiers in cellular neuroscience 2010 May 14;4:12. https://doi.org/10.3389/fncel.2010.00012
- 3.Diwakar S, Magistretti J, Goldfarb M, Naldi G, D’Angelo E. Axonal Na+ channels ensure fast spike activation and back-propagation in cerebellar granule cells. Journal of neurophysiology 2009 Feb;101(2):519-32. https://doi.org/10.1152/jn.90382.2008
- 4.Masoli S, Solinas S, D’Angelo E. Action potential processing in a detailed Purkinje cell model reveals a critical role for axonal compartmentalization. Frontiers in cellular neuroscience 2015 Feb 24;9:47. https://doi.org/10.3389/fncel.2015.00047
- 5.Reimann MW, King JG, Muller EB, Ramaswamy S, Markram H. An algorithm to predict the connectome of neural microcircuits. Frontiers in computational neuroscience 2015 Oct 8;9:28. https://doi.org/10.3389/fncom.2015.00120
P59 Reconstruction of effective connectivity in the case of asymmetric phase distributions
Azamat Yeldesbay1, Gereon Fink2, Silvia Daun2
1University of Cologne, Institute of Zoology, Cologne, Germany; 2Research Centre Jülich, Institute of Neuroscience and Medicine (INM-3), Jülich, Germany
Correspondence: Azamat Yeldesbay (azayeld@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P59
The interaction of different brain regions is supported by transient synchronization between neural oscillations at different frequencies. Different measures based on synchronization theory are used to assess the strength of the interactions from experimental data, e.g. the phase-locking index, phase-locking value, phase-amplitude coupling, and cross-frequency coupling. Another approach measuring connectivity based on the reconstruction of the dynamics of phase interactions from experimental data was suggested by [3]. On the basis of this method and the theory of weakly coupled phase oscillators, [2] presented a variant of Dynamic Causal Modelling (DCM) for the analysis of phase-coupled data, where a Bayesian model selection and inversion framework is used to identify the structure and directed connectivity among brain regions from measured time series.
Most of the research on phase analysis relies on the direct association of the phases of the signals with the phases used in the theoretical description of weakly coupled oscillators. However, [1] showed that the phases of the signals measured in experiments are not uniquely defined and an asymmetric distribution of the measured phases (e.g. non-sine form of the signals) could result in a false estimation of the effective connectivity between the network nodes. Furthermore, [1] suggested a solution for this problem by introducing a transformation from an arbitrarily measured phase to a uniquely defined phase variable.
In this work we merge the ideas from the Dynamical Causal Modelling by [2] with the phase dynamics reconstruction by [1] and present a new modelling part that we implemented into DCM for phase coupling. In particular, we extended it with a distortion (a transformation) function that accommodates departures from purely sinusoidal oscillations.
By numerically analysing synthetic data sets with an asymmetric phase distribution, generated from models of coupled stochastic phase oscillators and coupled neural mass models, we demonstrate that the extended DCM for phase coupling with the additional modelling component correctly estimates the coupling functions that do not depend on the distribution of the observables.
The new proposed extension of DCM for phase coupling allows for different intrinsic frequencies among coupled neuronal populations, thereby making it possible to analyse effective connectivity between brain regions within and between different frequency bands, to characterize m:n phase coupling, and to unravel underlying mechanisms of the transient synchronization.
References
- 1.Kralemann B, Cimponeriu L, Rosenblum M, Pikovsky A, Mrowka R. Phase dynamics of coupled oscillators reconstructed from data. Physical Review E. 2008 Jun 9;77(6):066205.
- 2.Penny WD, Litvak V, Fuentemilla L, Duzel E, Friston K. Dynamic causal models for phase coupling. Journal of neuroscience methods 2009 Sep 30;183(1):19–30.
- 3.Rosenblum MG, Pikovsky AS. Detecting direction of coupling in interacting oscillators. Physical Review E. 2001 Sep 21;64(4):045202.
P60 Movement related synchronization affected by aging: A dynamic graph study
Nils Rosjat, Gereon Fink, Silvia Daun
Research Centre Jülich, Institute of Neuroscience and Medicine (INM-3), Jülich, Germany
Correspondence: Nils Rosjat (n.rosjat@fz-juelich.de)
BMC Neuroscience 2019, 20(Suppl 1):P60
The vast majority of motor actions, including their preparation and execution, is the result of a complex interplay of various brain regions. Novel methods in computational neuroscience allow us to assess interregional interactions from time series acquired with in-vivo techniques like electro-encephalography (EEG). However, our knowledge of the functional changes in neural networks during non-pathological aging is relatively poor.
To advance our knowledge on this topic, we recorded EEG (64 channels) from 18 right-handed healthy younger subjects (YS, 22–35 years) and 24 right-handed healthy older subjects (OS, 60–79 years) during a simple motor task. The participants had to execute visually-cued low frequency left or right index finger tapping movements. Here, we used the relative phase-locking value (rPLV) [1] to examine whether there is an increase in functional coupling of brain regions during this simple motor task. We analyzed the connectivity for 42 electrodes focusing on connections between electrodes lying above the ipsi- and contralateral premotor and sensorimotor areas and the supplementary motor area.
Widely used approaches for network definition are based on certain functional connectivity measures (e.g. similarity in BOLD time series, phase locking, coherence). These methods typically focus on constructing a single network representation over a fixed time period. However, this approach cannot make use of the high temporal resolution of EEG data and is not able to shed light on the understanding of temporal network dynamics. Here we used graph theory-based metrics that were developed in the last several years that can deal with the analysis of temporally evolving network structures [2].
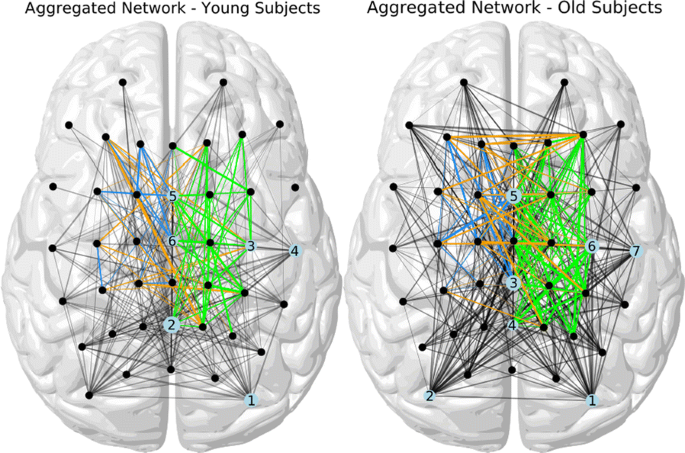
Our rPLV network analysis revealed four major results: An underlying coupling structure around movement onset in the low frequencies (2–7 Hz) that is present in YS and OS. The network in OS involved several additional connections and showed an overall increased coupling structure (Fig. 1). While the motor related networks of YS mainly involved ipsilateral frontal, contralateral frontal and central electrodes and interhemispheric pairs of electrodes connecting frontal ipsilateral with central contralateral ones, the networks of OS showed especially an increased interhemispheric connectivity. The analysis of hub nodes and communities showed a strong involvement of occipital, parietal, sensorimotor and central regions in YS. While the networks of OS involved similar hub nodes, the first occurrence of sensorimotor regions was clearly delayed and central electrodes played a more important role in the network (Fig. 1). Moreover, the motor related node degrees were significantly increased in OS.
Aggregated networks for younger (left) and older subjects (right) summarizing the network connectivity over the whole time interval. Edges lying above the motor cortex are highlighted in blue (ipsilateral), green (contralateral) and orange (interhemispheric). Hub nodes are marked in the order of first appearance scaled by their frequency
In addition to previously published results [3, 4], we were able to unravel the time-development of specific age-related dynamic network structures that seem to be a necessary prerequisite for the execution of a motor act. The increased interhemispheric connectivity of frontal electrodes fits very well to previous fMRI literature reporting an overactivation in frontal regions in older subjects. Our results also hint at a loss of lateralization via increased connectivity in both hemispheres as well as interhemispheric connections.
References
- 1.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human brain mapping 1999, 8(4), 194–208.
- 2.Sizemore AE, Bassett DS. Dynamic graph metrics: Tutorial, toolbox, and tale. NeuroImage 2018, 180, 417-427.
- 3.Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. The handbook of aging and cognition 2008, 3, 1-54.
- 4.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and aging 2002, 17(1), 85.
P61 How a scale-invariant avalanche regime is responsible for the hallmarks of spontaneous and stimulation-induced activity: a large-scale model
Etienne Hugues, Olivier David
Université Grenoble Alpes, Grenoble Institut des Neurosciences, Grenoble, France
Correspondence: Etienne Hugues (etienne.hugues@upf.edu)
BMC Neuroscience 2019, 20(Suppl 1):P61
At rest, BOLD fMRI and MEG recordings have revealed the existence of functional connectivity (FC) [1] and of scale-invariant neural avalanches [2], respectively. Under stimulation, neural activity is known to propagate on the brain network and, across trials, firing variability is found to be generically reduced [3]. Understanding the properties of the spontaneous state emerging on the brain network, together with its modifications during stimulation is a fundamental problem in neuroscience, still largely untouched.
A large-scale modeling approach, where the brain network is modeled by local neuronal networks connected through the large-scale connectome, has been previously used. Assuming that the whole brain is in an asynchronous state, the noisy fluctuations reverberating on the network have been found to be responsible for BOLD FC. However, in this fluctuation scenario, stimulation-induced activity is strongly damped while propagating on the network, even when trying to correct for this limitation [4].
We show that low spontaneous firing prevents neural activity to propagate in the fluctuation scenario. Adding neural adaptation, a local node can have two dynamical states, allowing the network dynamics to escape from the fluctuation regime, through brief excursions of individual nodes towards the higher activity state, allowing neural activity to effectively propagate on the network.
In the spontaneous state, the model exhibits neural avalanches, whose size distribution is scale-invariant for some global coupling strength value. BOLD FC is found to originate from the avalanches, therefore from nonlinear dynamics. The best agreement with empirical BOLD FC is found for scale-invariant avalanches.
Stimulation tends to entrain some nodes towards the high activity state, eliciting a reproducible propagation on the network, which simultaneously leads to a decrease of neural variability compared to the spontaneous state where more fluctuations occur, attributing a global origin to this phenomenon. Finally, neural activity is found to propagate optimally in the scale-invariant avalanche regime.
In conclusion, this study demonstrates that, beyond the brain connectome, a spontaneous state in the scale-invariant avalanche regime is crucial to reproduce the hallmarks of spontaneous and stimulation-induced activity. Neural variability decreases wherever activity propagates reliably, going beyond experimental results [3] and previously proposed mechanisms. Overall, the present work proposes a unified theory of the large-scale brain dynamics for a wide range of experimental findings.
Acknowledgments: We thank the European Research Council for supporting E.H. and O.D. with E.U.’s 7th Framework Programme / ERC Grant 616268 “F-TRACT”.
References
- 1.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience 2007, 8, 700–711.
- 2.Shriki O, Alstott J, Carver F, et al. Neuronal avalanches in the resting MEG of the human brain. Journal of Neuroscience 2013, 33, 7079–7090.
- 3.Churchland MM, Yu BM, Cunningham JP, et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nature Neuroscience 2010, 13, 369–378.
- 4.Joglekar MR, Mejias JF, Yang GR, et al. Inter-areal balanced amplification enhances signal propagation in a large-scale circuit model of the primate cortex. Neuron 2018, 98, 222–234.
P62 Astrocytes restore connectivity and synchronization in dysfunctional cerebellar networks
Paolo Bonifazi1, Sivan Kanner2, Miri Goldin1, Ronit Galron2, Eshel Ben Jacob3, Ari Barzilai2, Maurizio De Pitta’4
1Biocruces Health Research Institute, Neurocomputational imaging, Bilbao, Spain; 2Tel Aviv University, Department of Neurobiology, Tel Aviv, Israel; 3Tel Aviv University, School of Physics and Astronomy, Tel Aviv, Israel; 4Basque Center for Applied Mathematics: BCAM, Bilbao, Spain
Correspondence: Paolo Bonifazi (paol.bonifazi@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P62
In the last two decades, it has become appreciated that glial cells play a critical role in brain degenerative diseases (BDDs). The symptoms of BDDs arise from pathological changes to neuro-glia interactions, leading to neuronal cell death, disrupted neuro-glia communication, and impaired cell function, all of which affect global dynamics of brain circuitry. Astrocytes, a particular glial cell type, play key roles in regulating pathophysiology of neuronal functions. In this work, we tested the hypothesis that neuronal circuit dynamics were impacted as a consequence of disrupted neuron-astrocyte physiology in a mouse model of the BDD that results from a deficiency in the ATM protein. The gene encoding ATM is mutated in the human genetic diseaseAtaxia-Telangiectasia (A-T). One of the most devastating symptoms of A-T is the cerebellar ataxia, with significant loss of Purkinje and granule neurons in the cerebellum, that leads progressively to general motor dysfunction. We used primary cerebellar cultures grown from postnatal wild-type (WT) and Atm −/− mice to study how ATM deficiency influences the structure and dynamics of cerebellar neuronal-astrocyte circuits. We hypothesized that ATMdeficiency impairs the neuronal-astrocytic interactions underlying spontaneous neuronal synchronizations, a hallmark activity pattern of the developing nervous system.
We report that the absence of Atm in neurons and astrocytes severely alters astrocyte morphology and the number of pre- and post-synaptic puncta, disrupting the topology and dynamics of cerebellar networks. Functionally, Atm −/− networks showed a reduced number of global synchronizations (GSs) which recruited the whole imaged neuronal population, in favor of an increased number of sparse synchronizations (SSs), where only a small subset of neurons of the network fired in together. Structurally, higher numbers of synaptic puncta in Atm−/−networks relative to numbers in wild-type cultures were associated with lower levels of autophagy. These reported structural and functional anomalies were all rescued in chimeric neuronal networks composed of Atm−/−neurons and WT astrocytes. In contrast, cultures of WT neurons with Atm−/−astrocytes led to significant neuronal cell death. Characterizations of adult Atm−/−cerebella similarly showed disrupted astrocyte morphology, upregulated GABAergic markers, and dysregulated mTOR-mediated signaling and autophagy.
The apparent contradiction between a larger number of synapses in the Atm−/−circuits and lower occurrence of network synchronizations could result from the presence of non-functional connections (aborted functional connectivity hypothesis) or from the homeostatic downscaling of synaptic weights between neurons (aborted effective connectivity hypothesis). We explore the latter hypothesis extrapolating on its possible consequences on in-vivo cerebellar dynamics. With this regard we presenta spiking neural network model for the above described in-vitro experiments, where increase in connectivity in parallel withscaling of synaptic weightscan account for the increase of SSs in the KO model. Next, we consider the same increase in connectivity yet in relation to GABAergic transmission in a simplified model of cerebellar circuits and we show that an increase of inhibitory connections results in a reduction of functional connections in evoked excitatory activity, suggesting disrupted sensory and motor processing cascade in ataxia.
P63 Pybrep: Efficient and extensible software to construct an anatomical basis for a physiologically realistic neural network model
Ines Wichert1, Sanghun Jee2, Sungho Hong3, Erik De Schutter3
1Champalimaud Center for the Unknown, Champalimaud Research, Lisbon, Portugal; 2Korea University, College of Life Science and Biotechnology, Seoul, South Korea; 3Okinawa Institute of Science and Technology, Computational Neuroscience Unit, Okinawa, Japan
Correspondence: Sungho Hong (shhong@oist.jp)
BMC Neuroscience 2019, 20(Suppl 1):P63
In building a physiologically realistic model of a neural network, one of the first challenges is to determine the positions of neurons and their mutual connectivity based on their anatomic features. Recent studies have shown that cell locations are often distributed in non-random spatial patterns [1–3]. Also, synaptic and gap junction-mediated connectivity is constrained by the spatial geometry of axonal and dendritic arbors. These features have to be taken into account for realistic modeling since they determine convergence/divergence of the input/output of the neurons, respectively, and fundamentally impact their spatiotemporal activity patterns [4,5].
Here we present pybrep, an easily usable and extendable Python tool, designed for efficient generation of cell positions and connectivity based on anatomical data in large neuronal networks, and demonstrate its successful application to our previously published network model of the cerebellar cortex [5] and its extension. In a first step, pybrep generates cell positions by the Poisson disk sampling algorithm [6]: By sampling quasi-random points in a space with a constraint on their mutual distances, it simulates tight packing of spherical cells with given radii. We adapted this to generate multiple cell types sequentially and apply coordinate transformations to compensate for anisotropic geometry. Based on those locations, it generates point clouds representing specified axonal and dendritic morphologies. Using an efficient nearest neighbor search algorithm, it then identifies candidate connections by finding points that satisfy a distance condition. This can be done in 3D, or in some cases even more efficiently with a 2D projection method that exploits morphological regularities such as the long parallel fibers in the cerebellar network.
In the setup process for the cerebellar cortex model, pybrep efficiently produced the positions of more than a million cellular structures, including granule and Golgi cells as well as mossy fiber glomeruli, based on existing data about densities, volume ratios, etc. [7] Notably, applying a physiologically plausible, distance-based connection rule to the generated positions reproduced the well-known 4-to-1 connectivity between glomeruli and granule cells [7]. Pybrep also generated synaptic connectivity, particularly between the granule and Golgi cells, by an order of magnitude faster compared to our previous software for the same task [5]. Finally, the modular structure of pybrep allowed for an easy extension of the existing model by adding a new cell type, the molecular layer interneuron.
Pybrep depends only on a few external packages, but can easily be combined with existing Python tools, such as those for parallelization and scaling-up. These features will make pybrep a useful tool for constructing diverse network models in various sizes.
References
- 1.Töpperwien M, van der Meer F, Stadelmann C, Salditt T. Three-dimensional virtual histology of human cerebellum by X-ray phase-contrast tomography. PNAS 2018 Jul 3;115(27):6940–5.
- 2.Jiao Y, Lau T, Hatzikirou H, Meyer-Hermann M, Corbo JC, Torquato S. Avian photoreceptor patterns represent a disordered hyperuniform solution to a multiscale packing problem. Physical Review E 2014 Feb 24;89(2):022721.
- 3.Haruoka H, Nakagawa N, Tsuruno S, Sakai S, Yoneda T, Hosoya T. Lattice system of functionally distinct cell types in the neocortex. Science 2017 Nov 3;358(6363):610–5.
- 4.Rosenbaum R, Smith MA, Kohn A, Rubin JE, Doiron B. The spatial structure of correlated neuronal variability. Nature neuroscience 2017 Jan;20(1):107.
- 5.Sudhakar SK, Hong S, Raikov I, et al. Spatiotemporal network coding of physiological mossy fiber inputs by the cerebellar granular layer. PLoS computational biology 2017 Sep 21;13(9):e1005754.
- 6.Bridson R. Fast Poisson disk sampling in arbitrary dimensions. In SIGGRAPH sketches 2007 Aug 5 (p. 22).
- 7.Billings G, Piasini E, Lőrincz A, Nusser Z, Silver RA. Network structure within the cerebellar input layer enables lossless sparse encoding. Neuron 2014 Aug 20;83(4):960–74.
P64 3D modeling of complex spike bursts in a cerebellar Purkinje cell
Alexey Martyushev1, Erik De Schutter2
1Okinawa Institute of Science and Technology (OIST), Erik De Schutter Unit, Onna-son, Okinawa, Japan; 2Okinawa Institute of Science and Technology, Computational Neuroscience Unit, Onna-Son, Japan
Correspondence: Alexey Martyushev (martyushev.alexey@gmail.com)
BMC Neuroscience 2019, 20(Suppl 1):P64
The cerebellum regulates motor movements through the function of its Purkinje neurons. Purkinje neurons generate electrophysiological activity in the form of firing simple (fast) and complex (slow) spikes differing in the number of spikes, amplitude and duration. The interest in studying the complex spike bursts is based on their role in controlling and learning human body movements.
This study describes a new version of the recently published spatial single Purkinje cell model implemented in the NEURON simulation software by [1]. This model uses a variety of ionic mechanisms to generate simple and complex spike activity. We analyze the difference in modeling results between the NEURON [2] and the Stochastic Engine for Pathway Simulation (STEPS) [3] simulation environments. The NEURON modeling approach idealizes the complex 3D morphology as cylinders (>10 µm scale) with uniform membrane properties and considers only 1D membrane potential propagation, while STEPS treats the neuron morphology in the form of a more detailed (<1 µm scale) tetrahedral 3D mesh [3]. These differences affect channel properties and calcium dynamics in the Purkinje cell model. Additionally, the need of detailed neuronal modeling leverages the increase of using electron microscopy to provide super resolution neuronal reconstructions.
The results of this study will detail our understanding of intrinsic properties and functioning of neurons at the nanoscale. Possible differences between the two software tools may require us to reconsider our approaches to computational modelling of the neuronal activity in the brain [4].
References
- 1.Zang Y, Dieudonne S, De Schutter E. Voltage- and Branch-Specific Climbing Fiber Responses in Purkinje Cells. Cell Rep. 2018, 24(6), p. 1536–1549.
- 2.Carnevale NT, Hines M. The NEURON Book. Cambridge, UK: Cambridge University Press; 2006.
- 3.Hepburn I, et al. STEPS: efficient simulation of stochastic reaction-diffusion models in realistic morphologies. BMC Syst Biol. 2012, 6, p. 36.
- 4.Chen W, De Schutter E. Time to Bring Single Neuron Modeling into 3D. Neuroinformatics 2017, 15, p. 1–3.
































No hay comentarios:
Publicar un comentario