
Dasatinib Approved by FDA for Some Children with Chronic Myelogenous Leukemia
November 30, 2017, by NCI Staff
On November 9, the Food and Drug Administration (FDA) approved dasatinib (Sprycel®) for the treatment of some children with chronic myelogenous leukemia (CML). The approval is for children with CML whose cancer cells have a genetic change known as the Philadelphia chromosome and whose disease is in a relatively early stage, known as the chronic phase.
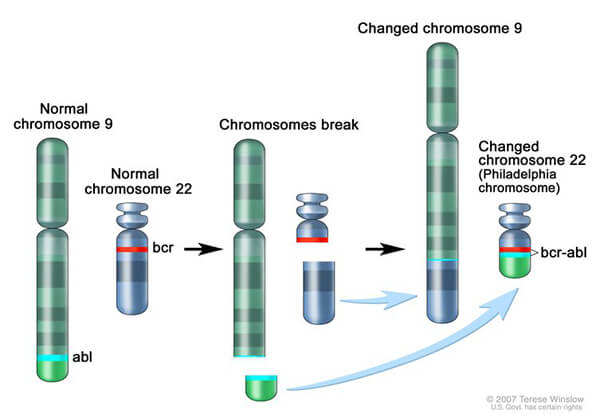
Most cases of CML are caused by the Philadelphia chromosome, which is created when a piece of chromosome 9 and a piece of chromosome 22 break off and trade places. This change results in the production of a fusion protein, called BCR-ABL, which drives the production of too many white blood cells, a hallmark of the disease.
Dasatinib and a drug called imatinib (Gleevec®) both inhibit BCR-ABL. Dasatinib may be effective in people who can no longer benefit from imatinib or who cannot take the drug because of side effects.
FDA based its approval of dasatinib on results from two early-phase clinical trials—a phase 1 study and a phase 2 study—that included a total of 97 children with CML. The studies were funded by Bristol-Myers Squibb.
Fifty-one of the 97 patients—all from the phase 2 trial—had newly diagnosed CML. The remaining 46 patients (17 from the phase 1 trial and 29 from the phase 2 trial) could not tolerate imatinib or had CML that was resistant to imatinib.
After 24 months of treatment, 96.1% of patients newly diagnosed with CML and 82.6% of patients whose CML could not be treated with or had stopped responding to imatinib (Gleevec®) had a complete response.
In the phase 2 trial, “the responses occurred relatively quickly, and patients continued to derive benefit from the treatment the longer they stayed on the trial,” said study co-investigator Lia Gore, M.D., of the University of Colorado Anschutz Medical Campus.
The finding that patients who could not benefit from imatinib may respond to dasatinib was also important, noted study co-investigator C. Michel Zwaan, M.D., Ph.D., of Erasmus MC in the Netherlands.
Moreover, Dr. Zwaan continued, “the treatment was remarkably well tolerated. Typical side effects that occur in adults, such as pleural effusions, were hardly seen in the pediatric population.”
The side effects reported in more than 10 percent of the patients included headache, nausea, diarrhea, skin rash, vomiting, pain in an extremity, abdominal pain, fatigue, and joint pain.
After a median follow up of 4.5 years in newly diagnosed patients and 5.2 years in the patients who had previously been treated with imatinib, more than half of the patients had not experienced disease progression. As a result, the researchers cannot yet estimate the median duration of complete responses.
Because not all children can swallow tablets, the form of dasatinib used in adults, the trials used a liquid form of dasatinib that could be taken once a day in a single dose.
Dr. Zwaan noted that this group of patients could be taking the drug for decades and that researchers would need to follow the patients to understand the long-term safety of the treatment.
“There is always concern about long-term side effects, particularly for children who may require very long-term therapy,” added Dr. Gore. “As a community, we will need to continue to assess and follow these patients to ensure that the best short- and long-term balances of risks and benefits are achieved.”
































No hay comentarios:
Publicar un comentario