

| Clinical Trials | ||
| Updates from the National Cancer Institute | ||
| Clinical Trials News | ||
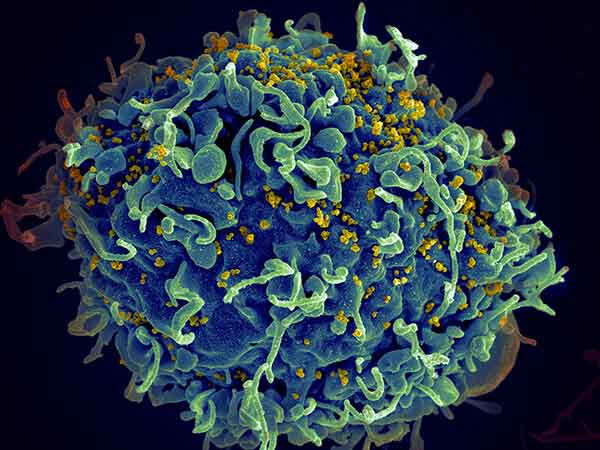 | Expanding Cancer Clinical Trial Access for Patients with HIV People with HIV are often excluded from cancer clinical trials because of concerns that the treatments may be unsafe for them. Preliminary results from an NCI-sponsored study of an immunotherapy drug show that people with HIV can safely participate in clinical trials. | |
 | FDA Approves Alectinib For Initial Treatment of ALK-Positive Lung Cancer The Food and Drug Administration (FDA) has approved alectinib (Alecensa®) as a first-line treatment option for patients with advanced non-small cell lung cancer (NSCLC) that is ALK positive. The approval was based on results from a phase 3 clinical trial of 303 patients with ALK-positive NSCLC who were randomly assigned to receive alectinib or crizotinib (Xalkori®), the first ALK inhibitor approved to treat ALK-positive NSCLC. | |
 | Dasatinib Approved by FDA for Some Children with Chronic Myelogenous Leukemia On November 9, the FDA approved dasatinib (Sprycel®) for the treatment of children with chronic myelogenous leukemia (CML) whose cancer cells express the Philadelphia chromosome and whose disease is in a relatively early stage, known as the chronic phase. FDA based its approval on results from two early-phase clinical trials—a phase 1 study and a phase 2 study—that included a total of 97 children with CML. | |
 | Find NCI-Supported Clinical Trials Use our search form to find a clinical trial or other research study that may be right for you or a loved one. | |
| Clinical Trials Information for Patients and Caregivers | ||
| Get Help Finding NCI-Supported Clinical Trials This page explains how to use NCI’s clinical trials search form to find NCI-supported clinical trials and review the results of your searches. It includes important points to consider when searching for clinical trials, instructions for using the basic and advanced search forms, information to help you read and understand your search results, and instructions on how to connect with the NCI Contact Center for help. | ||
| What Are Clinical Trials? Clinical trials are research studies in which some type of intervention is given to people. They play a critical role in making progress against all types of cancer. This page provides basic information about clinical trials and why they are important. | ||
| NCI-Supported Clinical Trials that Are Recruiting Patients | ||
| Antibody Treatment for Children and Young Adults with Solid Tumors This phase 1 trial studies the safety and efficacy of enoblituzumab in children and young adults with solid tumors that express a protein called B7-H3, which helps regulate T cells. Eligible tumors include neuroblastoma, rhabdomyosarcoma, osteosarcoma, Ewing sarcoma, Wilms tumor, and others. | ||
| Combination Kinase Inhibitors for Patients with Relapsed or Resistant B-cell Cancers This phase 1/2 trial will assess the safety, tolerability, and dosage of vistusertib combined with acalabrutinib for patients with B-cell malignancies that have not responded to or have recurred after previous treatments. | ||
| Targeted Drug Therapy for Pancreatic Cancer That Has Spread This phase 2 trial will test the efficacy of selumetinib in patients with locally advanced or metastatic pancreatic cancer that has KRAS G12R mutations. Selumetinib may inhibit the activity of MEK 1 and 2, essential enzymes in a molecular pathway often implicated in cancer cell progression. | ||






















.png)









No hay comentarios:
Publicar un comentario