
Advancing the Potential and Promise of Total-Body PET Imaging
April 7, 2017, by Paula Jacobs, Ph.D., and Antonio Sastre, Ph.D.
For many people, the story of the National Institutes of Health (NIH) is about biomedical research—supporting and conducting research on the inner workings of cells and biological systems, better understanding diseases, and developing new therapies. And, indeed, advancing biomedical research is at the heart of NIH’s mission.
But there’s another important part of the story of NIH that is often underappreciated: its role in technology development. A powerful new type of PET scanner under development is an ideal example of the role that NIH institutes like ours—NCI and the National Institute of Biomedical Imaging and Bioengineering (NIBIB)—play in helping to bring new research and patient care-related technologies to the market.
This new PET scanner, whose development has received substantial financial support from NCI and NIBIB, is designed to rapidly provide detailed information about tissues throughout the entire body while using a much smaller amount of radioactivity than a standard PET scan. The researchers working on this effort—led by Simon Cherry, Ph.D., and Ramsey Badawi, Ph.D., of the University of California, Davis—recently reported that they are on track to have the first working prototype of this total-body PET (TB-PET) scanner completed by next year.
TB-PET scanners would transform what is possible with PET imaging, creating a host of applications that could fundamentally alter drug development and patient care, particularly for people with cancer.
There is undoubtedly a tremendous amount of work to be done before this transformation becomes a reality. But the progress made to date is not only encouraging but is illustrative of how NIH can aid in bringing potentially transformative technologies to research labs and hospitals across the country and world.
Supporting Translation and Transformation
Those who are familiar with the work of NIBIB are likely to understand and appreciate its important role in promoting the development of advanced imaging and other technologies. From a disease perspective, the institute is agnostic: NIBIB promotes the development of technologies that can improve people’s health and well-being, no matter whether they have cancer, an autoimmune disease, or have been in a terrible car accident.
That work extends from supporting the development of new technologies for imaging with unprecedented detail inside the body, making surgeries less invasive and more effective, and establishing advanced research models, to name just a few.
Some in the cancer community may not be familiar with NCI’s involvement in technology development. Although it’s not something that’s always front and center, it’s a well-established niche in the institute’s portfolio.
For example, NCI’s Small Business Innovation Research and Technology Transfer Research programs have a strong tradition of helping businesses advance technologies and therapies developed by NCI researchers or bring their own innovative technologies, including new research tools and advanced diagnostics, to market.
NCI’s Cancer Imaging Program (CIP) has also been instrumental in supporting the development of new technologies. One example is CIP’s support for the development of a CT imaging device designed exclusively for imaging studies of women at high risk of breast cancer that produces 3D images without requiring uncomfortable breast compression.
And CIP also supported the early trials and regulatory studies for fluciclovine F18 (Axumin®), a radiotracer agent for use in PET scans that was recently approved by the Food and Drug Administration to detect prostate cancer recurrence.
For both of our institutes, the support of TB-PET is a logical and exciting extension of our role in promoting the development of new medical technologies.
High-Risk, High-Reward
In 2015, NCI and NIBIB agreed to join with NIH Office of the Director to provide the funding needed to advance the TB-PET scanner to the prototype stage. Our institutes were able to provide that support through an NIH Transformative Research Award.
Although industry or venture capital groups often provide backing to support the development of new technologies, investors can be reluctant to back high-risk projects—even those with the promise of TB-PET. But it’s because of the potentially groundbreaking nature of this new PET technology that NCI and NIBIB agreed to join together to provide funding to support the Transformative Research Award grant proposal submitted by Dr. Badawi and his colleagues.
The research group launched their effort, called EXPLORER, in 2011. In the ensuing years, they made important progress toward creating the scanner’s design and understanding what it would take to make TB-PET a reality.
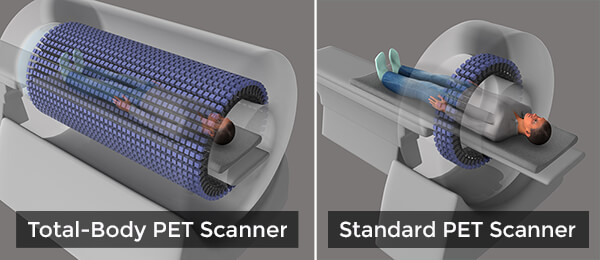
This new device would be a sea change from what is possible with current PET scanners, which can image only a small section of the body at a time in a procedure that can take 20–30 minutes and often requires the person being scanned to lie still in an uncomfortable position for the entire scan.
The linchpin of the TB-PET scanner is its ability to capture far more of the photon “signal” released from the radioactive tracers injected into the body. It is these tracers that allow PET scanners to create images of tissues and cellular processes. The TB-PET scanner will have a 6-foot long, open tube that is studded with 560,000 advanced sensors for picking up these photon signals.
Dr. Cherry and his team estimate that this robust signal capture will reduce scan times to an astounding 30 seconds or less—meaning they could be accomplished with patients only having to hold a single breath—while increasing the scanner’s ability to detect very small metastatic tumors or track whether a therapy is hitting its intended target, to cite just two potential uses.
This increased sensitivity should mean that far lower doses of radioactive tracers will be required for a scan, potentially reducing the radiation dose to patients by nearly 40 fold.
In fact, the extensive signal capture provided by TB-PET means that, in some cases, delivering a single dose of radioactive tracer may be sufficient for follow-up scans in a patient days or even weeks later.
The reduction in radiation dose is particularly important for people being treated for cancer—especially children, who are particularly susceptible to the DNA-damaging effects of radiation. Because of concerns about both acute adverse effects and late effects (including second cancers), pediatric oncologists often limit the use of PET when possible, opting for less sensitive imaging options in some cases.
The dramatically lower radiation doses that will be possible with TB-PET would substantially decrease the risks of PET scanning. And the rapid scan times would mean that babies and toddlers with cancer—or, indeed, with many other serious conditions or injuries—would not have to be anesthetized to undergo a PET scan (a procedure that also carries some risk), but could instead simply be swaddled to keep them still.
Adults, of course, could also benefit from reduced radiation doses and the reduced scan time. A 20–30 second scan, for example, means not having to hold both arms above the head for an extended period—a challenging proposition for many people and, for those who have difficulty staying still, one that can often impair image quality.
A Long-Term Commitment
As the research community and industry continue to pioneer the development of advanced technologies, NCI, NIBIB, and other NIH institutes will continue to be part of that process.
As for the TB-PET scanner, we’re extremely hopeful that the prototype scanner will be ready in 2018 and that the investigators leading its development and their collaborators can begin the process of testing how best to use it to advance clinical research and patient care.
We’re greatly encouraged by what we’ve seen thus far. And we’re hopeful that our support will help to bring about a truly transformational advance.























.png)









No hay comentarios:
Publicar un comentario