Clinical development of targeted and immune based anti-cancer therapies
Abstract
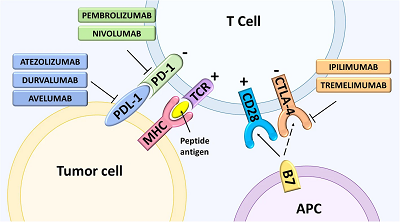
Cancer is currently the second leading cause of death globally and is expected to be responsible for approximately 9.6 million deaths in 2018. With an unprecedented understanding of the molecular pathways that drive the development and progression of human cancers, novel targeted therapies have become an exciting new development for anti-cancer medicine. These targeted therapies, also known as biologic therapies, have become a major modality of medical treatment, by acting to block the growth of cancer cells by specifically targeting molecules required for cell growth and tumorigenesis. Due to their specificity, these new therapies are expected to have better efficacy and limited adverse side effects when compared with other treatment options, including hormonal and cytotoxic therapies. In this review, we explore the clinical development, successes and challenges facing targeted anti-cancer therapies, including both small molecule inhibitors and antibody targeted therapies. Herein, we introduce targeted therapies to epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), human epidermal growth factor receptor 2 (HER2), anaplastic lymphoma kinase (ALK), BRAF, and the inhibitors of the T-cell mediated immune response, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1)/ PD-1 ligand (PD-1 L).
































No hay comentarios:
Publicar un comentario