
FDA Clears Wider Use of Cooling Cap to Reduce Hair Loss during Chemotherapy
July 21, 2017, by NCI Staff
The Food and Drug Administration (FDA) has cleared a cooling cap—a device designed to reduce hair loss during chemotherapy—for use by patients with any kind of solid tumor. FDA initially cleared the device, the DigniCap® Scalp Cooling System, for patients with breast cancer in 2015.
The expanded clearance of DigniCap is for “reducing the frequency and severity of hair loss” in adult patients with solid tumors who are receiving chemotherapy types and doses that are associated with this common side effect, the agency explained in a statement.
Some types of chemotherapy can cause hair on the scalp—as well as on other parts of the body—to fall out. Although hair loss caused by chemotherapy is usually temporary, many patients with cancer consider it one of the most distressing side effects of treatment. “Hair loss can be so distressing that some people avoid getting the recommended treatment,” said Dawn Hershman, M.D., of the Columbia University Herbert Irving Comprehensive Cancer Center, who wrote a recent editorial about two clinical trials testing scalp cooling devices.
Scalp cooling, which has been used in Europe for several decades, is thought to prevent hair loss by reducing blood flow to hair follicles, Dr. Hershman noted. Cooling the scalp causes blood vessels to constrict, which may limit the amount of chemotherapy drug that reaches hair follicles.
The idea of cooling the scalp to prevent hair loss has been around since the 1970s. Early efforts involved putting ice packs on the scalps of patients undergoing chemotherapy. More recent approaches have used cooling caps that are chilled and need replacing periodically during a session to maintain cold.
Testing the Device
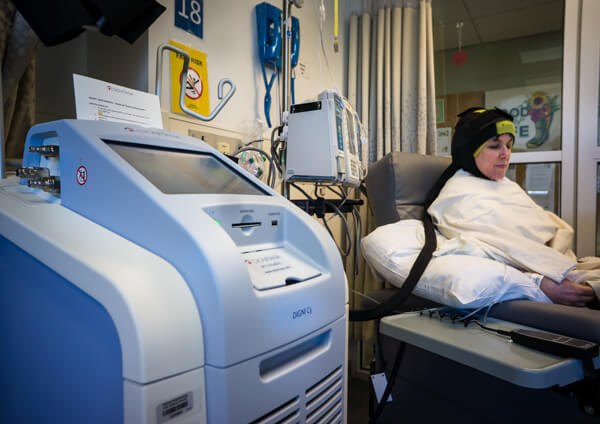
The DigniCap system uses a tightly fitted cap in which cold liquid circulates to cool the scalp before, during, and after chemotherapy. This cap, which is connected to a machine that regulates the cooling process, is covered by an outer cap, made of neoprene, that acts as an insulator.
FDA’s expanded clearance was based in part on a study involving 226 patients with various types of solid cancer who received different types of chemotherapy. Of this group, 146 (65%) had no hair loss or mild hair loss, and only 63 (28%) opted for a wig, according to the study results.
The researchers reported that the effectiveness of scalp cooling depended on various factors, including the chemotherapy regimen received. Although there has been some concern that scalp cooling could trigger increased metastases to the scalp, the researchers saw no evidence of that—a finding that was supported by a recent review of studies on scalp cooling and the risk of scalp metastases.
FDA’s original clearance for DigniCap was based on results published in JAMA from an observational study of 122 patients who received non-anthracycline-based chemotherapy for early-stage breast cancer. Of the 101 participants who had scalp cooling, 5 (4%) experienced no hair loss and 62 (61.4%) had less than 50% hair loss, whereas all 16 participants in the control group had more than 50% hair loss.
The most common side effects of the scalp cooling system included headaches induced by the cold, neck and shoulder discomfort, and pain associated with wearing the cooling cap for extended periods.
Expanding the Benefits
In an interview, Dr. Hershman said the expanded clearance might allow more patients to benefit from scalp cooling, as some women with breast cancer have in recent years.
“Scalp cooling should be offered to the appropriate patients who are receiving a chemotherapy regimen that has been tested with scalp cooling,” said Dr. Hershman. But she cautioned that patients should know that there’s still a chance they could lose their hair. “Scalp cooling is not 100% effective,” she added.
Citing the cost of treatment, Dr. Hershman noted that scalp cooling should not be offered to patients who are receiving chemotherapy indefinitely.
The average total cost of scalp cooling ranges between $1,500 and $3,000 per patient, depending on the number of cycles of chemotherapy. Insurance does not currently cover scalp cooling treatments, according to the maker of DigniCap, Dignitana Inc., of Sweden.
A Second Device
A second device, the Paxman Scalp Cooling System, has been evaluated in a randomized clinical trial and is under consideration by FDA.
In the study, women who used the Paxman Scalp Cooling System were significantly more likely to have less than 50% hair loss after the fourth cycle of chemotherapy than participants who did not have scalp cooling. All 47 women in the control group had more than 50% hair loss, whereas among the participants who received scalp cooling 5 (5%) had no hair loss and 43 (45%) had less than 50% hair loss. The study results also appeared in JAMA.
The introduction of new types of cancer treatments, such as targeted therapies, raises the prospect of “a future in which chemotherapy is no longer necessary and some of the distressing adverse effects of cancer treatments can be avoided,” wrote Dr. Hershman in her editorial in JAMA.
But until that time, she continued, interventions such as scalp cooling that reduce or eliminate toxic effects associated with cancer treatments “will help ease the distress associated with chemotherapy and may, as a result, improve outcomes for patients with breast cancer.”





















.jpg)










No hay comentarios:
Publicar un comentario