From Guideline Database
This database contains updated guidelines, policies and recommendations on genomic research and practice, as provided by professional organizations, federal advisory groups, expert panels and policy groups.
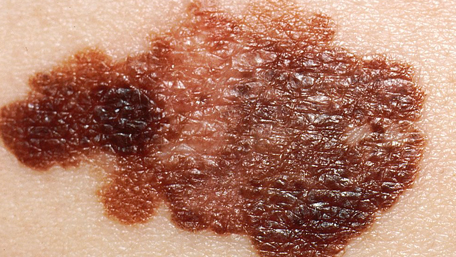
- FDA approval summary: vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation.

Published 2014 - Guidelines for biomarker testing in metastatic melanoma: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology.

Published 2014 ( Spanish Society of Pathology) ( Spanish Society of Medical Oncology) - Vemurafenib for treating locally advanced or metastatic BRAF V600 mutation-positive malignant melanoma - (NICE technology appraisal guidance [TA269])

Published 2012 ( National Institute for Health and Care Excellence) - A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment.

Published 2015 ( American College of Medical Genetics and Genomics) ( National Society of Genetic Counselors)






















.png)









No hay comentarios:
Publicar un comentario