04/13/2017 | |
Two studies have identified proteins that drive growth of diffuse intrinsic pontine glioma (DIPG) tumor cells. Blocking these targets with investigational drugs slowed tumor growth in animal models. | |

Studies Identify Potential Treatment Strategies for Pediatric DIPG Brain Tumors
April 13, 2017, by NCI Staff
Two separate studies have identified potential therapeutic targets in an inoperable pediatric brain tumor, diffuse intrinsic pontine glioma (DIPG). Blocking these targets with investigational drugs slowed tumor growth in animal models of DIPG.
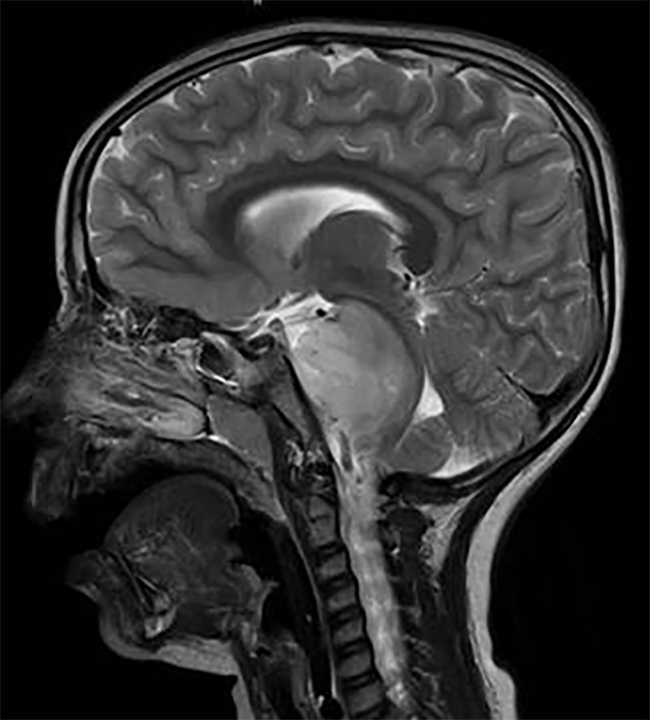
DIPG, a tumor that is located in the brain stem, is almost uniformly fatal.
"As a pediatric neurooncologist, it's one of the most devastating tumors we see," said Pratiti Bandopadhayay, M.B.B.S., Ph.D., of the Dana-Farber/Boston Children's Cancer and Blood Disorders Center, who was not involved in the studies. "We have no curative treatments at all for these tumors."
While the majority of DIPG tumors carry a specific genetic mutation, it was previously unclear what role, if any, the mutant protein plays in tumor development, and whether its function could be targeted by therapies.
For both new studies, the research teams investigated the biology of human DIPG cells with this mutation and identified characteristics that may make them vulnerable to treatment. Compared with control treatments, they found, drugs known as PRC2 and BET inhibitors shrank DIPG tumors in mouse models and lengthened the mice's lives.
The results of both studies, one from a research group at Northwestern University and another from a group at the University of Copenhagen in Denmark, were published February 27 in Nature Medicine.
Identifying a Target
About 5 years ago, researchers working on the St. Jude–Washington University Pediatric Cancer Genome Project discovered that nearly 80% of DIPG tumors have a specific mutation in the gene for a protein called histone H3.
That such a high percentage of tumors have this same mutation came as a surprise to the DIPG research community, said Ali Shilatifard, Ph.D., of the Northwestern University Feinberg School of Medicine, lead investigator for one of the studies.
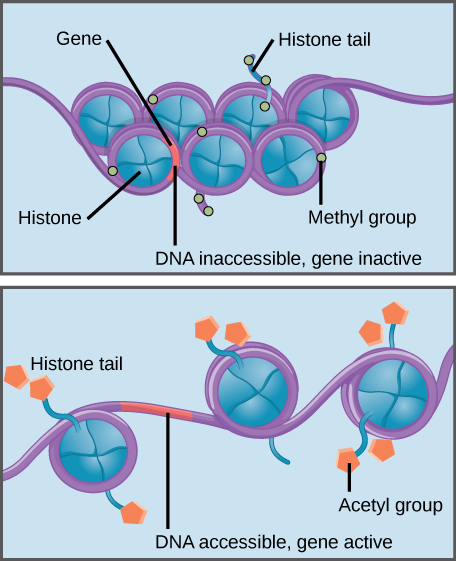
Histones are a family of proteins that help package DNA into compact structures. Short sections of DNA wind around histone proteins like thread on a spool, and thousands of DNA–wrapped histones (called nucleosomes) make up each chromosome.
Specific modifications to histones can promote or prevent gene expression. For example, the attachment of chemical compounds called acetyl groups to histones weakens their interaction with DNA, promoting gene expression. On the other hand, the addition of methyl groups to histones usually makes DNA wind more tightly around histones, preventing gene expression.
Identifying the presence of a histone H3 mutation in patients with DIPG was the first step, said Dr. Bandopadhayay. "But to be able to treat tumors with that alteration, you have to understand what the mutation is doing," she added.
In an earlier study, Dr. Shilatifard and his colleagues used fruit flies to study the function of the mutant histone H3 gene. Compared with flies that had normal histone H3 proteins, flies with the mutant version had more histones that were studded with acetyl groups (acetylated). In turn, these acetylated histones were bound by molecules called bromodomain-containing (BRD) proteins, which regulate gene expression.
For their current study, which was funded in part by NCI, the Northwestern researchers wanted to determine whether the mutant histone had the same function in human cells. Indeed, they found that human colon cancer or kidney cancer cells altered to express the mutated histone H3 gene had more acetylated histones than cells that expressed the normal histone H3 gene.
The first author, Andrea Piunti, Ph.D., then examined all the mutant H3 histones located throughout the genomes of DIPG tumor cells from three patients. His analysis revealed that many nucleosomes that contained mutant histones were acetylated and bound by BRD proteins.
Additional experiments showed that BRD proteins may play a direct role in DIPG tumor growth. Treating human DIPG cells with investigational drugs that block BRD proteins, called BET inhibitors, slowed DIPG cell growth compared with a control treatment. And in studies of mice with human DIPG cells implanted into their brain stems, those treated with BET inhibitors had smaller tumors and lived longer than mice treated with the control treatment.
These experiments "suggest that BET inhibitors provide a potential therapeutic approach for DIPG," said Dr. Shilatifard. Preclinical studies have shown that several different cancer types—including leukemia and glioblastoma—are also sensitive to BET inhibitors, he added.
An Additional Target
The Northwestern group's analysis of human DIPG tumor cells with mutant histone H3 also revealed that in addition to more histone H3 acetylation, many histones were decorated with methyl groups (methylated). Furthermore, they found that an enzyme called PRC2—which attaches methyl groups to histone H3 proteins—was present near many of these histones.
These results prompted the Northwestern researchers to look further into PRC2 activity. They found that blocking PRC2 activity—either genetically or with a PRC2 inhibitor called tazemetostat—reduced DIPG cell growth.
"Unexpectedly, these results demonstrate a role for PRC2 function in the maintenance of DIPG growth," the Northwestern group wrote.
The Copenhagen group also found evidence supporting PRC2's role in promoting DIPG tumor growth. They observed that tazemetostat and another PRC2 inhibitor reduced the growth of mouse brain cells or human DIPG cells with mutant histones, whereas the control treatment did not.
And when they implanted mouse brain cells with mutant histones into the brains of normal mice or mice in which PRC2 was genetically blocked, those that lacked PRC2 activity lived longer.
In contrast, however, a separate study by a research group in Germany found that cells from different patients with DIPG were not sensitive to tazemetostat, even though their tumors had the histone H3 mutation.
A Step Forward
Decades of clinical trials have shown that DIPG tumors are insensitive to traditional chemotherapy, explained Dr. Bandopadhayay. And although radiation can alleviate symptoms, it’s not curative, and most children with DIPG die within 2 years of diagnosis.
A Different Histone-Modifying Drug
In 2015, an international consortium of DIPG researchers banded together to study all available DIPG cell samples. Their study revealed that DIPG cells collected from several different patients were killed by a histone-modifying drug called panobinostat (Farydak®). Panobinostat blocks enzymes that add acetyl groups to histones. Now researchers, who are part of the NCI-supported Pediatric Brain Tumor Consortium, are testing the safety and best dose of panobinostat for children with DIPG in a phase I clinical trial.
In 2015, an international consortium of DIPG researchers banded together to study all available DIPG cell samples. Their study revealed that DIPG cells collected from several different patients were killed by a histone-modifying drug called panobinostat (Farydak®). Panobinostat blocks enzymes that add acetyl groups to histones. Now researchers, who are part of the NCI-supported Pediatric Brain Tumor Consortium, are testing the safety and best dose of panobinostat for children with DIPG in a phase I clinical trial.
Now results from both studies point to potentially promising new treatment strategies, Dr. Shilatifard said.
"We want to move BET inhibitors into a phase Istudy for the treatment of DIPG, and our colleagues at the Ann & Robert H. Lurie Children's Hospital of Chicago are pushing forward with those studies," he said.
In addition, several ongoing clinical studies are testing tazemetostat, the PRC2 inhibitor, in both adult and pediatric patients with various cancers. However, no current study is specifically investigating tazemetostat in children with DIPG.
There are still many unanswered questions, said Dr. Bandopadhayay, including whether these investigational drugs have the ability to cross the blood–brain barrier and reach human brain tumors.
Dr. Bandopadhayay and her colleagues are also learning more about DIPG biology by studying tumor cells obtained from biopsies, in addition to those obtained from autopsies. Obtaining biopsy samples has been made possible only recently by major improvements in neurosurgical techniques, she explained.
"It's an exciting time," she said. "There's a lot of work underway right now. The ultimate goal is to be able to treat these kids so that they have a chance of cure with minimal side effects."























.png)









No hay comentarios:
Publicar un comentario