
Crizotinib Shows Promise for Childhood Cancers
September 5, 2017, by NCI Staff
Children with cancers that have alterations in the ALK gene may benefit from the targeted drug crizotinib (Xalkori®), according to updated results from a clinical trial.Tumors shrank in most of the children in the trial, and some of these tumor responses have lasted for more than 2 years, researchers reported in the Journal of Clinical Oncology on August 8.
The trial enrolled 26 children with relapsed and difficult-to-cure: anaplastic large cell lymphoma(ALCL), which is an aggressive type of non-Hodgkin lymphoma, and a rare soft tissue sarcoma known as an inflammatory myofibroblastic tumor (IMT).
“Crizotinib is a very effective drug” for pediatric patients with ALK alterations, said Yael P. Mossé, M.D., of the Children’s Hospital of Philadelphia, who led the clinical trial. “The drug is highly active, and the responses were durable and meaningful.”
The NCI-supported Children’s Oncology Group (COG) Phase I Consortium conducted the trial, called ADVL0912, in collaboration with Pfizer, which manufactures and supplied crizotinib. Earlier results from the study were published in 2013.
“This is an important pediatric precision medicine clinical trial,” said Malcolm Smith, M.D., Ph.D., of NCI’s Cancer Therapy Evaluation Program. “It shows that high response rates can be achieved when an effective targeted agent like crizotinib is matched to a patient population whose cancers have the relevant genomic alterations.”
Gene Fusions Fuel Tumor Growth
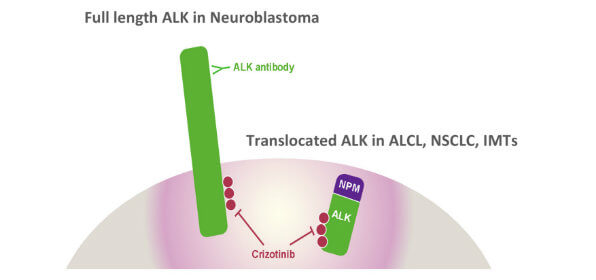
Crizotinib blocks growth-promoting messages through the ALK signaling pathway. The drug is approved for patients with non-small cell lung cancer whose tumors have certain alterations in the ALK gene or the ROS1 gene.
One type of genomic alteration occurs when a segment of the ALK gene breaks off and fuses with part of another gene. Such gene fusions result in abnormal proteins that can drive uncontrolled cell growth, leading to tumors.
Anaplastic large cell lymphomas account for up to 15% of childhood lymphomas, and most of these tumors carry the ALK gene fusion. Abnormalities in the ALK gene occur in about half of inflammatory myofibroblastic tumors.
In the trial, the rate, type, and duration of responses exceeded those typically seen in early-phase clinical trials involving children with relapsed or refractory cancers, the study authors noted. One possible reason, they suggested, is that ALK signaling is the primary driver of cell growth in these two pediatric cancers.
“ALK provides the fuel for the growth of these tumors,” said Dr. Mossé. “The tumors are entirely ‘addicted’ to ALK signaling.” By contrast, she continued, ALK signaling is just one of multiple factors that contribute to cell growth in lung cancer and other types of tumors.
Strong Tumor Responses to Crizotinib
The study evaluated two different doses of crizotinib in patients with lymphoma. The overall response rate was 83% for patients who received the lower dose and 90% for those who received the higher dose.
For patients with inflammatory myofibroblastic tumors a range of doses was tested, and the overall response rate was 86%.
In addition, the researchers observed a complete response in 83% of the lymphoma patients who received the lower dose, 80% of the lymphoma patients who received the higher dose, and 36% of the patients with inflammatory myofibroblastic tumors.
The partial response rates were 0% and 10% for the lymphoma patients at the lower and higher doses, respectively, and 50% for patients with inflammatory myofibroblastic tumors.
“The onset and durability of responses were not dose-dependent,” Dr. Mossé said, noting that objective and sustained responses were seen at the lower and higher doses in the lymphoma patients.
The most common side effect associated with treatment was a decrease in a type of immune cells called a neutrophil. Twelve patients with lymphoma stopped the therapy to undergo bone marrow transplantation at the discretion of their treating physicians.
Looking Ahead
The challenge now for researchers studying anaplastic large cell lymphoma is to determine how to incorporate crizotinib into the overall management of children with the disease, Dr. Smith noted.
The ongoing Children’s Oncology Group clinical trial ANHL12P1 is studying whether adding crizotinib or another targeted therapy, brentuximab (Adcetris®), to the standard chemotherapy regimen for ALCL can improve outcomes.
The ADVL0912 trial combined elements of phase I and phase II studies. First, the researchers identified a dose of crizotinib that could be safely used in children, and then they assessed the ability of crizotinib to shrink tumors in patients with alterations in the ALK gene who had the potential to benefit.
Developing trials that incorporate elements of both phase I and phase II studies “can be a more efficient and expeditious way to evaluate novel agents in children with cancer,” said Dr. Smith.
The study also illustrates the importance of having the right biomarker, which allows researchers to select the patients who are most likely to benefit from a treatment, added Dr. Mossé.
“We understand so much more about the targets and the drugs we’re testing in pediatric trials than [we did] in the past,” she continued. “It’s an exciting time. If you identify the key molecular vulnerability in a tumor, and if you have the right drug, you can really make a big difference for patients.”






















.png)









No hay comentarios:
Publicar un comentario